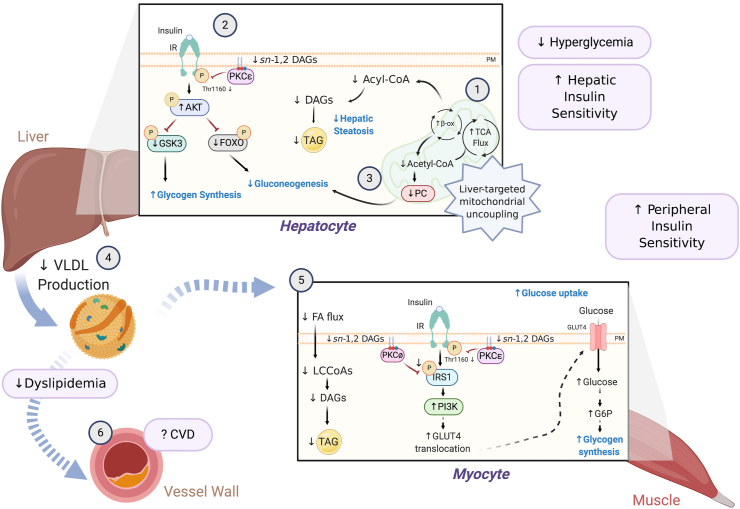
Figure 3.
Mechanism(s) by which liver-targeted mitochondrial uncouplers prevent metabolic syndrome. Liver-targeted mitochondrial uncoupling agents hold therapeutic promise for the treatment of MAFLD, NASH, and T2D by promoting increased hepatic cellular energy expenditure [1]. Uncoupling-mediated increases in hepatic fat oxidation lower hepatic triglycerides (TAGs), plasma membrane (PM) sn-1,2-DAG content, and PKCε translocation, which increase hepatic insulin sensitivity [2]. Subtle sustained increases in hepatic mitochondrial inefficiency also reduce hepatic acetyl-CoA content, pyruvate carboxylase (PC) activity, and gluconeogenesis [3]. Collectively, this leads to reduced fasting and postprandial hyperglycemia. Liver-targeted uncoupling also leads to reduced hepatic VLDL production [4], reducing intramyocellular plasma membrane sn-1,2-DAG content and PKCθ/PKCε activity and reversing muscle insulin resistance [5]. Overall, these improvements in dyslipidemia and whole-body insulin sensitivity in rodent and non-human primate models of MAFLD/NASH and T2D reduce the risk of developing atherosclerotic CVD [6] and suggest that liver-targeted mitochondrial uncoupling agents may be a viable therapy for treating cardiometabolic disease in humans.