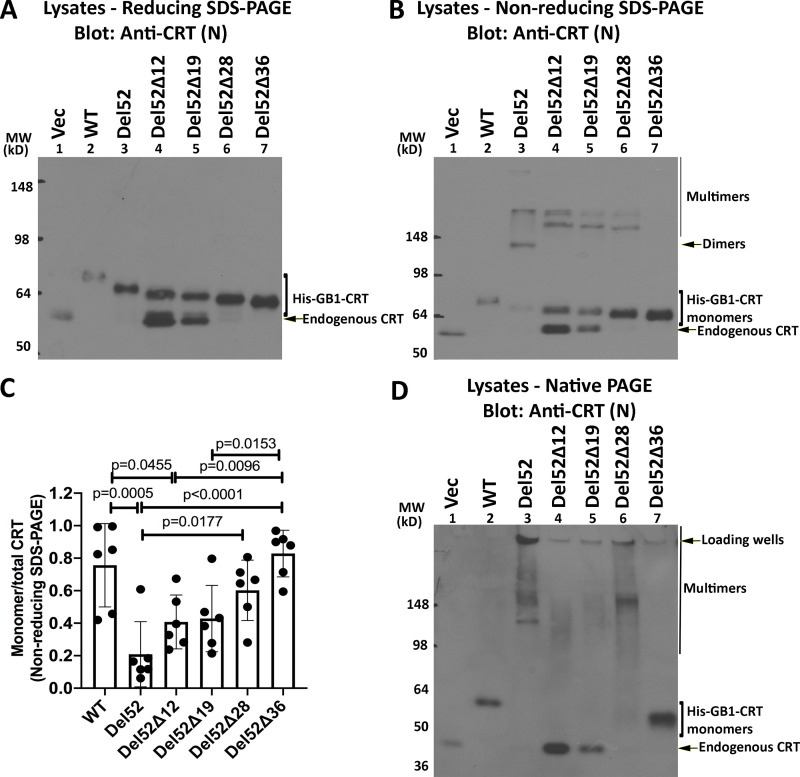
Figure 3.
Truncations of C-terminal cysteines of full-length CRTDel52 alter but do not abrogate disulfide-linked interactions. (A, B, and D) Lysates from HEK293T cells expressing N-terminal his-GB1–tagged full-length or C-terminally truncated CRTDel52 constructs were separated by SDS-PAGE under reducing (10% gels; A) or nonreducing (10% gels; B) conditions or by native-PAGE (4–20% gradient gels; D) and immunoblotted with the anti-CRT(N) antibody. Different amounts of lysates were loaded to achieve similar protein expression of different truncated constructs: CRTWT, 0.5 µg lysates; CRTDel52, 5 µg lysates; CRTDel52Δ12, 18 µg lysates; CRTDel52Δ19, 18 µg lysates; CRTDel52Δ28, 3 µg lysates; CRTDel52Δ36, 1.8 µg lysates; or a plasmid lacking CRT (Vec), 10 µg lysates. The endogenous CRT band serves as the lysate loading controls. Species consistent with the size of endogenous CRT, his-GB1-CRT monomers, dimers, multimers, and loading wells are indicated. (C) Quantification of CRT monomer/monomer + multimer (total) bands from B averaged over six independent blots from five independent transfections. Data show mean ± SD, with statistical significance assessed via ordinary one-way ANOVA. MW, molecular weight.