Abstract
Recent urban public water supply contamination events emphasize the importance of screening treated drinking water quality after distribution. In vitro bioassays, when run concurrently with analytical chemistry methods, are effective tools to evaluating the efficacy of water treatment processes and water quality. We tested 49 water samples representing the Chicago Department of Water Management service areas for estrogen, (anti)androgen, glucocorticoid receptor-activating contaminants and cytotoxicity. We present a tiered screening approach suitable to samples with anticipated low-level activity and initially tested all extracts for statistically identifiable endocrine activity; performing a secondary dilution-response analysis to determine sample EC50 and biological equivalency values (BioEq). Estrogenic activity was detected in untreated Lake Michigan intake water samples using mammalian (5/49; median: 0.21 ng E2Eq/L) and yeast cell (5/49; 1.78 ng E2Eq/L) bioassays. A highly sensitive (anti)androgenic activity bioassay was applied for the first time to water quality screening and androgenic activity was detected in untreated intake and treated pre-distribution samples (4/49; 0.93 ng DHTEq/L). No activity was identified above method detection limits in the yeast androgenic, mammalian anti-androgenic, and both glucocorticoid bioassays. Known estrogen receptor agonists were detected using HPLC/MS-MS (estrone: 0.72–1.4 ng/L; 17α-estradiol: 1.3–1.5 ng/L; 17β-estradiol: 1.4 ng/L; equol: 8.8 ng/L), however occurrence did not correlate with estrogenic bioassay results. Many studies have applied bioassays to water quality monitoring using only relatively small samples sets often collected from surface and/or wastewater effluent. However, to realistically adapt these tools to treated water quality monitoring, water quality managers must have the capacity to screen potentially hundreds of samples in short timeframes. Therefore, we provided a tiered screening model that increased sample screening speed, without sacrificing statistical stringency, and detected estrogenic and androgenic activity only in pre-distribution Chicago area samples.
Keywords: Effects-based method, estrogen, androgen, tapwater
Graphical Abstract
1. Introduction
Despite great strides in physical and chemical drinking water treatment over the last century (Cutler and Miller, 2005; Schoenen, 2002), the growing global human population has depleted many freshwater sources and increased reliance on effective water treatment processes (UNWAPP, 2017). In vitro effects-based methods (bioassays), when run concurrently with analytical chemistry methods, have gained popularity over the last decade as effective tools to evaluate the efficacy of water treatment processes (Conley et al., 2017b; Escher et al., 2014; Jia et al., 2015; Jia et al., 2016; Medlock Kakaley et al., 2020; Plewa and Wagner, 2015; Shi et al., 2018; Snyder and Leusch, 2018; Zhen et al., 2018). Much of the development and application of bioassays to water quality has focused on surface (Blackwell et al., 2019; Blackwell et al., 2017; Conley et al., 2017a; Jeong et al., 2012), waste (Dong et al., 2017; Dong et al., 2019; Leusch et al., 2018) and recycled water (Escher et al., 2014; Jia et al., 2015). In these water sample types, the detected biological activity can be relatively high. However, effects-based methods can also make exceptional tools for screening water samples that contain very low-level concentrations of individual contaminants (e.g. treated drinking water). This versatility results from bioassays’ inherent ability to detect cumulative biological activity from the sum of all present contaminants. Typically, biological activity in an environmental sample, reported in biological equivalency values (BioEq), is calculated by fitting a sigmoidal concentration-response curve to cell (or organism) responses from treatments of serial dilutions of a sample and determining an EC50 value and sample relative potency (compared to method reference compound). However, biological activity detected in treated drinking water can fall below an EC50 value, despite sample enrichment. Therefore alternative approaches to the traditional sigmoidal concentration-response curves to determining sample activity are necessary (Escher et al., 2018b).
In a preceding pilot study we sought to fill data gaps in chemical occurrences in point-of-use drinking water (tapwater, TW) using both bioassays and analytical chemical methods (Bradley et al., 2018). TW samples collected from locations across the United States suggested human exposure to mixtures of trace level organic and inorganic compounds that are not required to be monitored in TW. Herein we seek to identify potential sources, movement and transformation of biologically-active contaminants in treated drinking water during distribution. In a multi-agency collaborative study of pre- and post-distribution treated TW representing the Chicago Department of Water Management service areas, an extensive targeted-chemical toolbox (540 organic and 35 inorganic analytes) was employed and no detected chemical concentrations exceeded U.S. Environmental Protection Agency (USEPA) Maximum Contaminant Levels in any treated TW sample (Bradley et al., 2020). However, multiple exceedances of health-based advisories (e.g., maximum contaminant level goals; MCLG) in untreated and pre-distribution water samples, together with the recognized order of magnitude analytical underestimation in the TW exposure (350,000+ commercial compounds (Bradley et al., 2017) and unquantifiable transformation products (Dobson, 2004; Vasquez et al., 2014)), raised concerns for potential biological effects and therefore supported a non-targeted effects-based screening.
Bioassays that detect steroid hormone-active compounds have previously compensated for gaps in targeted analyte coverage (Bradley et al., 2017; Conley et al., 2017a; Medlock Kakaley et al., 2020). Therefore, we applied a suite of bioassays focused on steroid hormone signaling pathways to Chicago TW extracts to screen pre- and post-distribution water filtration plant (WFP)-treated water. Included in the suite was a highly sensitive method for detecting (anti)androgenic activity (Hartig et al., 2007) never before applied to water quality screening. Further, we provide a tiered screening model for sample analysis applicable to routine water quality monitoring that expedites drinking water sample extract evaluation while maintaining statistical stringency.
2. Methods
2.1. Sample Collection
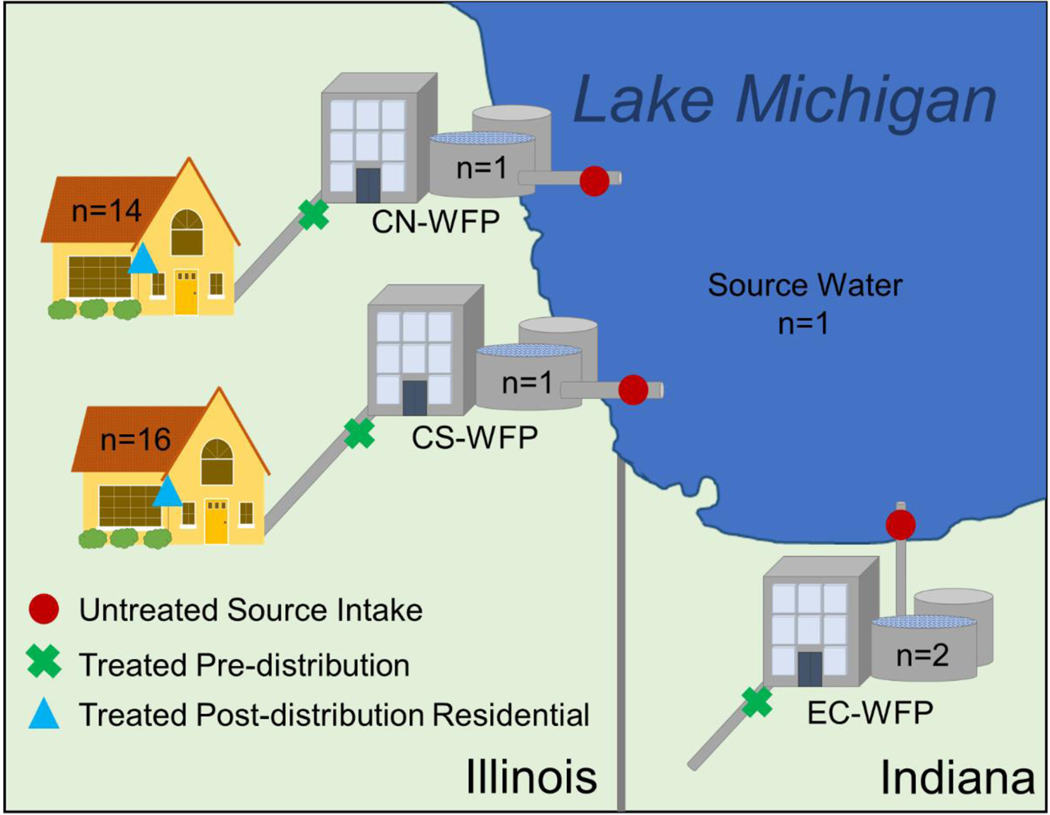
A total of 49 samples of raw-water intake from Lake Michigan (WFP-UT), WFP-treated/pre-distribution water (WFP-TW), and post-distribution tapwater (TW) from private residences (cold water samples without pre-cleaning, screen removal, or flushing; exact locations of private residences have been anonymized) were taken from Chicago, Illinois and East Chicago, Indiana between July and December 2017 using procedures that were previously described (Supporting Information) (Romanok et al., 2018). Sampling sites (38 total) were selected based on community volunteers and represented a broad geographical coverage of the City of Chicago Department of Water Management water filtration plants’ (WFP) service areas (Figure 1; Chicago North, CN: 16 sites; Chicago South, CS: 18 sites; East Chicago, EC: 4 sites). Only pre-distribution samples were screened from the City of East Chicago Utilities Department service area and were included for comparison to Chicago, IL pre-distribution samples. Field blanks were collected as part of the quality assurance and quality control protocols at three sites (CS-WFP-TW, EC-WFP-UT1 and EC-WFP-TW1). Sample aliquots from each sampling site were allocated for multiple analysis types e.g., analytical chemistry, endocrine bioassays and cytotoxicity bioassay.
Figure 1. Water sampling scheme.
Three sample types were taken from sites around the Greater Chicago metropolitan area including untreated Lake Michigan source water (red circle), water filtration plant (WFP)-treated pre-distribution water (green “x”), and post-distribution residential tapwater (blue triangle). Untreated intake and WFP-treated water was sampled at two WFPs in East Chicago, IN, one WFP in North Chicago, IL and one WFP in South Chicago. Residental post-distribution tapwater samples representing the North (n = 14) and South Chicago (n = 16) WFPs distribution areas were also sampled.
2.2. Analytical Chemistry
Water samples were analyzed by U.S. Geological Survey (USGS) using 14 organic (540 unique analytes) and 7 inorganic (37 analytes) analytical methods (Supporting Information) and a full report of detected analytes were reported previously (Bradley et al., 2020). Of the 550 analytes, several known estrogen, androgen, and glucocorticoid receptor agonists were quantified using HPLC/MS-MS (Yost et al., 2013; Yost et al., 2014), as described previously (Bradley et al., 2020; Romanok et al., 2018), and are reported and discussed herein.
2.3. Endocrine Bioassays
A 4 L sample from each site was extracted into 400 μL methanol using the solid phase extraction methods described previously (Supporting Information) (Romanok et al., 2018). Two 100 μL extract aliquots were shipped overnight on ice to both U.S. Environmental Protection Agency, Research Triangle Park, NC (mammalian bioassay) and USGS Leetown Science Center, Kearneysville, WV (yeast bioassay) for in vitro screening of steroid hormone activity.
2.3.1. Mammalian Bioassays
The T47D-KBluc cell line (American Type Cell Culture, ATCC, Manassas, VA; #CRL-2865) (Wilson et al., 2004; Wilson et al., 2002) has been applied to chemical and environmental sample testing for estrogenic activity previously (Conley et al., 2017a; Conley et al., 2017b). Cell culture maintenance and sample screening were conducted as previously described (Wilson et al., 2004), with exceptions (Supporting Information) (Medlock Kakaley et al., 2020). Briefly, treatments included methanol vehicle control, serially diluted water samples, 17-beta estradiol (E2; CAS#: 50-28-2; Sigma) standard curve or 1 μM ICI 182,780 (antagonist control; Tocris Bioscience) in competition with E2 in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% dextran-coated charcoal treated fetal bovine serum (DCC-FBS).
The CV1 cell line (ATCC; CCL-70), which is naturally devoid of glucocorticoid and androgen receptor (AR) was cultured and transduced with the human glucocorticoid receptor (GR) to test for glucocorticoid activity in water samples as previously described (Hartig et al., 2002; Medlock Kakaley et al., 2018). Approximately 5 × 106 cells were transduced with the human GR (Ad/GR4) and MMTV-luciferase (Ad/Luc7), with a multiplicity of infection (MOI) of 1 and 50, respectively. Cells were plated at a rate of 22,000-33,000 cells/well, and exposed to cell media with either the methanol vehicle control, serially-diluted water extract samples, dexamethasone standard curve (Dex; CAS#: 50-02-2; Sigma) or 300 nM mifepristone (antagonist control; CAS: 84371-65-3; Sigma) in competition with Dex for 24 hrs. CV1-cells were frozen at −80°C with lysis buffer and thawed at room temperature before analyzing with luminometer.
Using a similar CV1 transduction system, water extract samples were also tested for (anti)androgenic activity using a CV1-chAR bioassay (Hartig et al., 2007). Culturing, transduction and exposure methods are identical to the CV1-hGR bioassay with the following exceptions. Cells were transduced with chimpanzee androgen receptor (chAR) and the MMTV-luc gene with MOIs of 1 and 50, respectively. In the AR agonist assay cells were exposed to media with either methanol vehicle control, 4,5α-Dihydrotestosterone (DHT; CAS#: 521-18-6; Sigma) standard curve (0, 3, 10, 30, 100, 300, 1000, 3000 pM), hydroxyflutamide (OHF; antagonist control; CAS#: 52806-53-8; Sigma) in competition with DHT, or serially diluted extract samples. In the AR antagonist assay, standards and samples were diluted with a concentration of AR agonist that produces approximately 80% of the maximal response (100 pM DHT) in RMPI. Cells were exposed to media with either methanol vehicle control (no DHT), OHF standard curve concentrations (0, 1, 3, 10, 30, 100, 300, 1000, nM), or serially diluted water extract samples. Antagonist activity was quantified as OHF equivalents (OHFEq).
All cells for T47D-KBluc and CV1-hGR/chAR screening assays were plated in 96-well luminometer plates. Each standard, control, or sample was run in quadruplicate, and each sample screen was at least duplicated i.e. different cell passage number. After 24 hr in vitro exposure, cells were visually scored for cytotoxicity (scale of 1–5) and any wells with cells exhibiting cytotoxic effects were excluded from subsequent analysis (Bhatia and Yetter, 2008; Conley et al., 2017a). Cells were washed, lysed and immediately following injection of luciferase reaction buffer and Firefly D-luciferin substrate, luminescence was quantified by measuring luminescence every 0.2 sec for 5 sec using a FLUOstar luminometer (BMG Labtech, Cary, NC) (Medlock Kakaley et al., 2020). The method detection limits (MDL) for mammalian bioassays were determined using the equation described below and were as follows; 0.0044 ng E2Eq/L, 0.3 ng DHTEq/L, 67.10 ng OHFEq/L, and 1.06 ng DexEq/L for the T47KBluc, CV1-chAR, CV1-chAR in antagonist mode, and CV1-hGR bioassays, respectively.
2.3.2. Yeast Bioassays
To add depth to our in vitro analysis of endocrine activity in the water extract samples (Blackwell et al., 2019; Könemann et al., 2018; Medlock Kakaley et al., 2020), the bioluminescent yeast estrogen screen (BLYES; 490 BioTech, Knoxville, TN) also was used to assess estrogenicity (estrogen receptor-alpha only) in the water extract samples as previously described (Conley et al., 2017a; Sanseverino et al., 2005), with modifications (Ciparis et al., 2012). Extracts were also screened for androgenicity and glucocorticoid activity using yeast strains DSY-1555 and MCY-105, respectively, as previously described (Kassotis et al., 2016).
Complete method details are provided in the Supporting Information. Briefly, each assay plate included either a 17β-estradiol, DHT, or hydrocortisone standard curve, vehicle controls, and sample extracts. All treatments were added as 10 μL volumes to wells of solid white 96-well plates and methanol was allowed to evaporate to dryness prior to the addition of media and yeast reporter. Final sample dilutions were 1:20. The reference hormone/environmental contaminant-induced chemiluminescent signal was measured using a SpectraMax M4 microplate reader (Molecular Devices, San Jose, CA) in luminescent mode (100 ms integration time). Endocrine activity within each sample, relative to respective standard curves, was determined using a four-parameter logistic regression (SoftMax Pro 6.6.6, Molecular Devices) and corrected for sample enrichment. Method detection limits for the BLYES, DSY-1555, and DSY-105 bioassays were 0.10 ng/L 17β-estradiol, 1.3 ng/L dihydroxytestosterone, 1.1 ng/L hydrocortisone, respectively.
2.4. Cytotoxicity
The Chinese hamster ovarian (CHO) cell cytotoxicity assay measures the reduction in cell density (surrogate for viability) as a function of the concentration of the test (Supporting Information) (Plewa et al., 2002; Plewa et al., 2010; Wagner and Plewa, 2017). Water sample aliquots (20 L) were extracted into DMSO as described previously (Daiber et al., 2016). DMSO concentrate was diluted in F12+ 5% fetal bovine serum cell culture medium (F12-FBS). In a 96-well flat-bottomed microplate, a blank control (no cells), negative control (F12-FBS plus cells) and experimental samples were analyzed concurrently. Initially, all negative and experimental wells contained 3×103 CHO cells. A covered microplate was rocked at 37°C for 10 min, ensuring even cell distribution, and incubated for 72 hr at 37°C under 5% CO2. Cells fixed in methanol were stained with crystal violet and washed with DMSO: methanol (3:1 v/v)l. Fixed cells were incubated at room temperature for 10 min and subsequently analyzed at 595 nm with a SpectraMax™ microplate reader.
Detailed statistical methods for CHO cytotoxicity assay were conducted as described previously and are provided in the Supporting Information (Box et al., 1978; K and M, 2008). Experimental treatments were normalized to percent of negative control treatment (100% survival) and LC50 values were converted into mean cytotoxicity index (CTI) values (CTI = 103 × LC50−1) (Box et al., 1978; K and M, 2008). One-way analysis of variance (ANOVA) tests were conducted to determine the lowest concentration factor that induced cytotoxicity compared to negative control (P ≤0.05).
2.5. Screening Model and Data Analyses
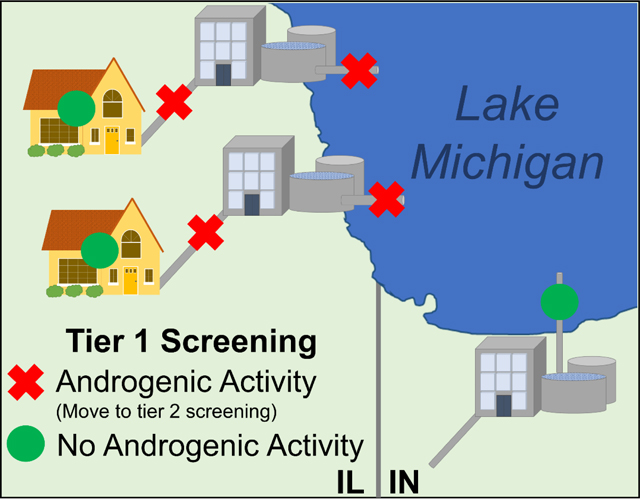
Steroid hormone activity is typically low, and often undetectable, in treated point-of-use TW, therefore we used a tiered screening process (Figure 2). In tier one, the presence of estrogenic/(anti)androgenic/glucocorticoid activity in each sample extract was determined. All 49 water sample extracts were screened at 10x and 5x final assay sample enrichment factors. Mean fold change above concurrent control of treatment replicates were normalized to percent maximal assay activity (based on assay-specific hormone standard saturation levels). Sample extracts with significant within plate increases/decreases in percent maximal activity of respective agonist/antagonist reference compound were identified with the General Linear Model Procedure using the Least Square Means Statement and Dunnett’s multiple comparison procedure.
Figure 2. Tiered screening and statistical analysis model.
for bioassays that measure endocrine activity in tapwater sample extracts. Here the model is presented using the CV1-chAR bioassay and Chicago area tapwater samples results. In tier one: Is there activity? all samples are screened with minimal dilutions and statistically compared to vehicle control (p > 0.05) using general linear model (GLM) and multiple comparison procedure (MCP). In tier two: How much activity? only active samples from tier one were screened again using a dilution-response to determine biological equivalency values (BioEq). Each sample BioEq is then compared to the bioassay method detection limit (MDL) and relevant trigger value.
In tier two, only active samples from tier one were screened using a series of 2-fold serial dilutions (1:1000, 1:2000, 1:4000, 1:8000, etc). Biological equivalency values (BioEq; reported in ng Reference/L) were calculated (Conley et al., 2017a; Medlock Kakaley et al., 2020) and graphically represented for each positive sample using the equation,
where the sample EC50 was a unitless bioassay sample dilution and was extrapolated using mean observed transcriptional activation values. The enrichment factor (EF) = 10,000 (prior to assay dilution) due to 4,000-fold concentration of sample during extraction (4 L water sample) and dried extract resuspension 0.4 mL methanol. Reference EC50 values are provided in Table S1.
The minimum detectable concentration (MDC) for hormone activity was determined as described previously (Conley et al., 2017b), and the method detection limit (MDL), where
and Final Sample EF = 10 (10,000x Enrichment Factor and 1,000x assay dilution), was used in the final step of the tiered screening process. Samples were identified as a positive hit for activity depending on whether the BioEq value was above or below the respective assay MDL.
2.6. Statistical Analysis
Statistical analyses were performed using SAS statistical software (Cary, NC USA) and graphs were generated using GraphPad Prism version 7.02 for Windows (GraphPad Software, LaJolla CA, USA).
3. Results
We efficiently screened raw WFP intake from Lake Michigan, WFP-treated pre-distribution water and point-of-use tapwater from locations near and around Chicago, IL (Table 1 and Figure 1), for multiple types of biological activity using a tiered testing model applicable to water quality monitoring (Figure 2). Overall, no treated point-of-use TW sample produced significant responses in endocrine activity (estrogenic, androgenic, anti-androgenic or glucocorticoid) or cytotoxicity. Estrogenic activity and androgenic activity were detected in raw WFP intake samples above method detection limits (Figure 3, 4 and Tables S2, S3, and S4).
Table 1.
Greater Chicago Area Sampling Site Locations, Dates, and Descriptions
| Site Identifier | Sample Date | Site Location/Description |
|---|---|---|
| CN-WFP-UT | July 2017 | Lake Michigan Intake |
| CN-WFP-UT | November 2017 | Lake Michigan Intake |
| CN-WFP-TW | July 2017 | Water Filtration Plant Pre-Distribution Effluent |
| CN-WFP-TW | November 2017 | Water Filtration Plant Pre-Distribution Effluent |
| CN-TW-1 | December 2017 | Residential Treated Tapwater |
| CN-TW-2 | November 2017 | Residential Treated Tapwater |
| CN-TW-3 | November 2017 | Residential Treated Tapwater |
| CN-TW-4 | December 2017 | Residential Treated Tapwater |
| CN-TW-5 | November 2017 | Residential Treated Tapwater |
| CN-TW-6 | November 2017 | Residential Treated Tapwater |
| CN-TW-7 | November 2017 | Residential Treated Tapwater |
| CN-TW-8 | December 2017 | Residential Treated Tapwater |
| CN-TW-9 | November 2017 | Residential Treated Tapwater |
| CN-TW-10 | November 2017 | Residential Treated Tapwater |
| CN-TW-11 | November 2017 | Residential Treated Tapwater |
| CN-TW-12 | November 2017 | Residential Treated Tapwater |
| CN-TW-13 | November 2017 | Residential Treated Tapwater |
| CN-TW-14 | December 2017 | Residential Treated Tapwater |
| CS-WFP-UT | July 2017 | Lake Michigan Intake |
| CS-WFP-TW | July 2017 | Water Filtration Plant Pre-Distribution Effluent (Field Blank) |
| CS-WFP-TW | July 2017 | Water Filtration Plant Pre-Distribution Effluent |
| CS-WFP-TW | November 2017 | Water Filtration Plant Pre-Distribution Effluent |
| CS-TW-1 | December 2017 | Residential Treated Tapwater |
| CS-TW-2 | December 2017 | Residential Treated Tapwater |
| CS-TW-3 | December 2017 | Residential Treated Tapwater |
| CS-TW-3 | December 2017 | Residential Treated Tapwater |
| CS-TW-4 | December 2017 | Residential Treated Tapwater |
| CS-TW-5 | December 2017 | Residential Treated Tapwater |
| CS-TW-6 | December 2017 | Residential Treated Tapwater |
| CS-TW-7 | December 2017 | Residential Treated Tapwater |
| CS-TW-8 | December 2017 | Residential Treated Tapwater |
| CS-TW-9 | December 2017 | Residential Treated Tapwater |
| CS-TW-10 | December 2017 | Residential Treated Tapwater |
| CS-TW-11 | December 2017 | Residential Treated Tapwater |
| CS-TW-12 | November 2017 | Residential Treated Tapwater |
| CS-TW-13 | November 2017 | Residential Treated Tapwater |
| CS-TW-14 | November 2017 | Residential Treated Tapwater |
| CS-TW-15 | November 2017 | Residential Treated Tapwater |
| CS-TW-16 | November 2017 | Residential Treated Tapwater |
| EC-WFP-UT1 | August 2018 | Lake Michigan Intake |
| EC-WFP-UT1 | September 2017 | Lake Michigan Intake |
| EC-WFP-UT1 | September 2017 | Lake Michigan Intake (Field Blank) |
| EC-WFP-TW1 | August 2018 | Water Filtration Plant Pre-Distribution Effluent |
| EC-WFP-TW1 | August 2018 | Water Filtration Plant Pre-Distribution Effluent (Field Blank) |
| EC-WFP-TW1 | September 2017 | Water Filtration Plant Pre-Distribution Effluent |
| EC-WFP-UT2 | August 2018 | Lake Michigan Intake |
| EC-WFP-UT2 | September 2017 | Lake Michigan Intake |
| EC-WFP-TW2 | August 2018 | Water Filtration Plant Pre-Distribution Effluent |
| EC-WFP-TW2 | September 2017 | Water Filtration Plant Pre-Distribution Effluent |
Each sampling event is identified with a three alpha-numeric element identifier; where the first element represents the geographical location (CN: Chicago North; CS: Chicago South; and EC: East Chicago, Indiana), the second indicates if the sample was collected pre-distribution (WFP: Water Filtration Plant) or post-distribution (TW), and the third indicates if the samples was untreated intake water (UT), or treated water (TW, or residence-specific numeric identifier). Two East Chicago WFPs were sampled and each East Chicago sample ends in a 1 or 2 indicating which plant was sampled.
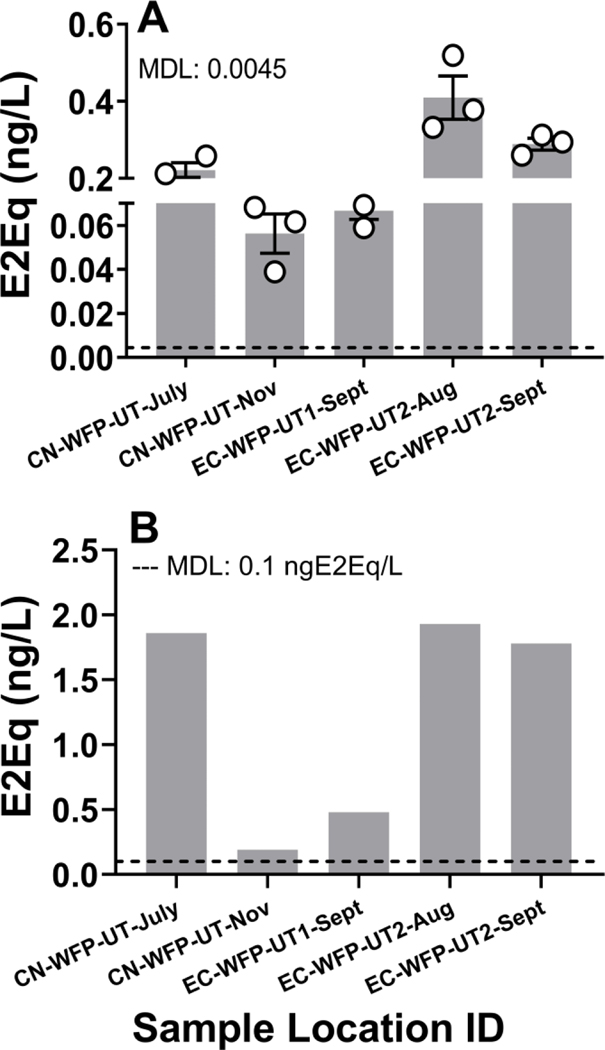
Figure 3. Estrogenic activity.
was measured in extract samples from untreated Lake Michigan intakes, WFP treated pre-distribution waters, and post-distribution waters using the A) T47D-KBluc (biological replicate data is shown as mean ± standard deviation) and B) BLYES bioassays. Method detection limit (MDL) for T47D-KBluc was 0.0044ng E2Eq/L and 0.1 ng E2Eq/L for BLYES assay.
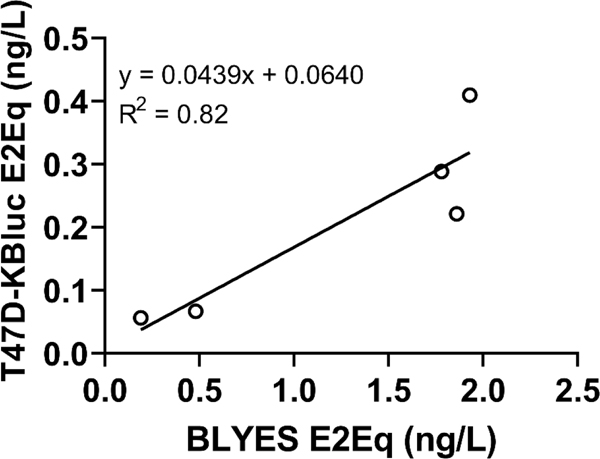
Figure 4. Estrogenic bioassay comparison.
using estrogenic activity detected in water sample extracts. T47D-KBluc and BLYES bioassays are compared through linear regression analysis where y = 0.0439x + 0.0640 and R2 = 0.82.
Estrogenic activity was detected in untreated WFP intake waters using both the T47D-KBluc and BLYES bioassays (Figure 3A and B). All five extract samples that produced responses above method detection limits for estrogenicity in the T47D-KBluc bioassay (MDL: 0.0044 ng estradiol equivalents(E2EqT47D-KBluc)/L) also produced positive responses in the BLYES bioassay (MDL: 0.10 ng E2EqBLYES/L). Estrogenic activity ranged from 0.04 to 0.52 ng E2EqT47D-KBluc/L (Mean: 0.21 ± 0.14 SD) and 0.19 to 1.93 ng E2EqBLYES/L (Mean: 1.24 ± 0.84). A linear regression analysis was conducted to compare the magnitude of estrogenic activity (ng E2Eq/L) in corresponding samples with positive detections. There was a distinct positive relationship between the results of the two bioassays (Figure 4; R2 = 0.82), although ng E2EqT47D-KBluc/L were consistently an order of magnitude lower than the corresponding ng E2EqBLYES/L.
In Bradley et al.(Bradley et al., 2020), many known estrogen receptor agonists were targeted environmental analytes and four were detected above established laboratory method reporting limits (Tables S5 and S6). Estrone (MRL: 0.5 ng/L) was detected in East (EC-WFP-TW1, Sept.) and South Chicago WFP-treated pre-distribution water (CS-WFP-TW, Nov.), one East Chicago WFP intake (EC-WFP-UT2, Sept.), and one South Chicago point-of-use samples extract (CS-TW-15). Both 17α-estradiol (MRL: 0.5 ng/L) and 17β-estradiol (MRL: 0.5 ng/L) were detected in one South Chicago pre-distribution water sample (CS-WFP-TW1, Nov.), and Equol (MRL: 0.5 ng/L), a non-steroidal phytoestrogen, was detected in a South Chicago point-of-use samples extract (CS-TW-14) (Table S6).
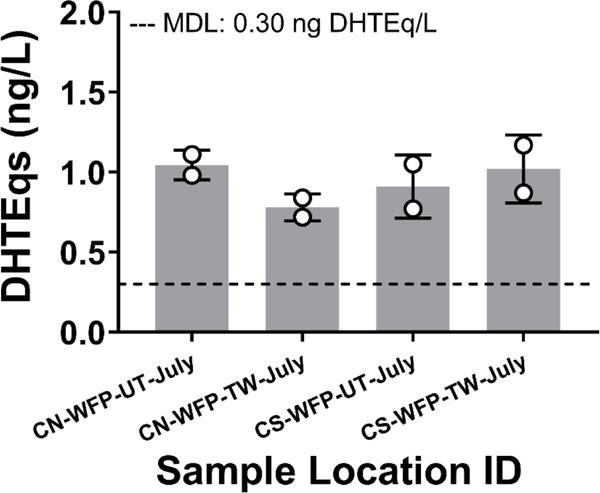
We used the inaugural application of an androgenic activity bioassay to illustrate a model for water quality monitoring with endocrine bioassays (Figure 2). Androgenic activity (significantly higher than vehicle control) was detected in two untreated intake and two treated pre-distribution water samples from North and South Chicago using the CV1-chAR bioassay (Figure 5). All four samples with quantifiable androgenic activity contained levels above the bioassay MDL (0.30 ng dihydroxytestosterone equivalents(DHTEq)CV1-chAR/L). Activity ranged from 0.77 to 1.17 ng DHTEqCV1-chAR/L (Mean: 0.94 ± 0.16), yet no sample produced androgenic activity above the yeast assay MDL for androgen activity (1.3 ng DHTEqDSY-1555/L). Further, of the targeted androgen receptor agonist analytes assessed, none were reported in the water extract samples above method detection limits by Bradley et al. (Table S5 and S6) (Bradley et al., 2020).
Figure 5. Androgenic activity.
was measured in extract samples from untreated Lake Michigan, WFP treated pre-distribution, and post-distribution waters using a CV1-chAR bioassay. Biological replicate data is shown as mean ± standard deviation and method detection limit (MDL) was 0.30 ng DHTEq/L.
The CV1-chAR bioassay can also be used in an antagonist mode, to evaluate the presence of compounds that might interfere with, or reduce, androgen receptor activity. No sample exhibited significant antagonist activity compared to the 100% maximal activity of 100 pM dihydrotestosterone positive control. The MDL for the CV1-chAR antagonist bioassay, 67.10 ng OHFEq/L, was calculated using the previously reported methods (Conley et al., 2017b), with the exception of using the lower 95% prediction interval around the 100% maximal 100 pM DHT response.
The CV1 cell line transduced with the human glucocorticoid receptor (CV1-hGR) can detect samples with glucocorticoid activity, but no samples contained significant activity compared to vehicle control (MDL: 1.06 ng DexEqCV1-hGR/L). Similarly, the chemiluminescent yeast bioassay for glucocorticoid activity (MDL: 1.1 ng DexEqMCY-105/L) did not produce any positive detections for glucocorticoid activity. Of the targeted glucocorticoid receptor agonist analytes assessed, none were reported in the water extract samples above method detection limits (Table S5 and S6) (Bradley et al., 2020).
Notably, the only sample with a significant increase in cytotoxicity from the CHO bioassay also produced measurable levels of estradiol equivalents in both estrogenic activity bioassays (EC-WF-UT1-Sept). Cytotoxicity (using cytopathogenic effect) was not apparent in either of the estrogenic bioassays (no reduction in cell viability, no reduction in bioluminescent signal) which may have resulted from variations in contaminant mixtures produced by the respective extraction methods.
4. Discussion
Drinking water treatment (Murray et al., 2019) and methodologies for water quality screening (Martin et al., 2007) continue to be developed and refined. However, isolated public water supply contamination events emphasize the value of specifically assessing treated drinking water after distribution from public water filtration plants (WFP). Aging water distribution infrastructure has contributed to altered water quality post-treatment in residential taps in Flint (2015) and Detroit, Michigan (2018) (Hyde, 2015). So in 2016, we conducted a pilot study to screen residential and workplace point-of-use tapwater samples across the US to investigate potential exposures from public water supplies (Bradley et al., 2018). The current wastewater and water infrastructure study described herein was designed to limit some of the variables present in the previous study. Only one source water, Lake Michigan, was used to determine the occurrence as well as the potential changes in chemical content of water during treatment and distribution. Further, the scope of water sampling was limited to a few water filtration plants and to residential sites served by those treatment facilities.
Despite the extensive list of targeted analytes included in the current study (complete list reported elsewhere (Bradley et al., 2020)), we (Bradley et al., 2017; Conley et al., 2017a; Medlock Kakaley et al., 2020) and others (Blackwell et al., 2019; Hashmi et al., 2020; Könemann et al., 2018) have previously shown that bioassays, which produce a cumulative value for all compounds affecting a single biological endpoint, may compensate for gaps in the targeted analytical chemical coverage. Könemann et al. reported estrogen activity detections two orders of magnitude lower compared to concentrations of targeted estrogens in European surface water sample analyzed using both methods (Könemann et al., 2018). However, bioassays are frequently applied to water quality monitoring using only relatively small samples sets often collected from surface and/or wastewater effluent (Escher et al., 2014; Neale et al., 2015; van der Oost et al., 2017).
To realistically adapt bioanalytical tools to treated water quality monitoring (especially in the absence of robotic sample and reagent dispensing), programs must have the capacity to screen potentially hundreds of samples in short timeframes, and subsequently obtain results in real time. Considering the number of samples tested in the current study and that we expected little to no detectable biological activity in many of the samples (30 tapwater samples), a tiered screening approach was preferable over the standard dilution-response testing. Locations within the US (Denison et al., 2020) and Europe (Brack et al., 2015) are already applying bioassays to water quality monitoring and the State of California’s guidance document for developing standard operating procedures for applying bioassays to water quality monitoring recommends a similar tiered approach to screening (Denison et al., 2020).
The tiered screening model we used is illustrated in Figure 2 using our inaugural application of the CV1-chAR bioassay as an example. To determine if androgenic activity was present, we screened all 49 samples using two sample dilutions and found only 4 samples with activity compared to concurrent plate vehicle control using the General Linear Model followed by Dunnet’s multiple comparison procedure. A secondary in vitro screen was conducted on the active samples only with a series of dilutions (concentration-response) to determine how much activity was in each sample. Using the DHT standard curves run concurrently with each sample, we determined all measurable activity in androgen active samples fell above the MDL. Although no human health EBTs have been determined for the CV1-chAR bioassay, all the samples fall below the previously reported EBTs for DHTEq established by Brand et al. for the AR-CALUX bioassay (11 ng DHTEq/L) in drinking water (Brand et al., 2013).
The addition of the bioassay screening components to the overall study design undoubtedly increased the spectrum of chemical detection. For example, in four of the five samples that produced estrogenic activity above MDL (CN-WFP-UT-July, CN-WFP-UT-Nov, EC-WFP-UT1-Sept, and EC-WFP-UT2-Aug; Figure 3A and B), none of the targeted estrogen receptor ligands were detected above analytical method detection limits. This could result due to the presence of 1) ER ligands that were not included in targeted analysis, 2) analytes included in the targeted analysis that have not been previously identified as an ER activating compound, or 3) a mixture of known and/or unidentified targeted ER ligands present at individual concentrations below analytical detection limits. Effects-directed analysis (EDA), an emerging experimental approach, could mitigate these recurring issues in scenarios one and two (Dong et al., 2020; Dusza et al., 2019; Hashmi et al., 2020; Zwart et al., 2020). An alternative explanation for the discrepancies in chemical and biological detection exists in the creation of sample aliquots and conducting separate extraction methods. While separate extraction methods are necessary to prevent contamination that interferes with biological analysis, separate methods create inherent bias i.e., extraction methods are optimized only to retain compounds that will be subsequently analyzed.
Estrogenic activity was detected in two of the four WFP intakes from Lake Michigan (T47D-KBluc), and was comparable to estrogenic activity previously detected upstream (0.14 ng E2EqT47D-KBluc/L) and downstream of wastewater treatment plants (0.16 ng E2EqT47D-KBluc/L) (Medlock Kakaley et al., 2020), and in European surface waters (0.16 ng E2EqHeLa/L) (Könemann et al., 2018). We have previously shown differences in resulting estradiol equivalents from the two estrogenic activity bioassays (Conley et al., 2017a), which may be due in part to T47D-KBluc measuring ERα and ERβ, and the BLYES bioassay measuring ERα activation only. Others assessing estrogenic activity with both human and yeast cell lines have also reported order of magnitude difference in maximum reported activity (Könemann et al., 2018). We have previously detected estrogenic activity in unfinished drinking water (pre-distribution) and treated drinking water above MDL at 0.03 (Medlock Kakaley et al., 2020) and 0.078 ng E2EqT47D-KBluc/L (Conley et al., 2017b), respectively. Estrogenicity has also been reported as high as 5.2 ng E2Eq/L in treated water in China using estrogenic activity bioassays other than the T47D-KBluc (Shi et al., 2018), but treated water from the Chicago area WFPs did not contain measurable levels of estrogenic activity.
Previous studies that have included bioanalytical and concurrent analytical chemistry methods have directly compared detected biological activity to the environmental concentrations of estrogens by transforming each environmental concentration to biological equivalency values using bioassay-specific compound potency (Conley et al., 2017a; Jia et al., 2016; Medlock Kakaley et al., 2020; Neale et al., 2015). This type of correlation analysis clarifies whether the targeted and detected environmental compounds capture the entire measured biological activity in each sample. However, only one sample, the September 2017 EC-WFP-UT2 water extract, produced a mean bioactivity of 0.29 ng E2Eq/L (Figure 3a) and contained 0.72 ng/L estrone (Table S6). The estimated T47D-KBluc bioactivity value, based on previously reported T47D-KBluc relative potency value for estrone (1.39) (Conley et al., 2016), was 1.00 ng E2Eq/L for the September 2017 EC-WFP-UT2 sample.
Imperative to the value of detected biological activity in treated tapwater is the exposure concentration at which it is predicted to adversely affect human health, e.g. effects-based trigger values (EBTs). Human health EBTs have been conceived using acceptable daily intakes, bioassay-specific relative potency factors, and pharmacokinetic parameters (Escher et al., 2018a). To our knowledge a T47D-KBluc specific-EBT has not been determined using either method. However, Brand et al. reported an EBT of 3.8 ng E2Eq/L for drinking water using a similar bioassay, ER-CALUX. Although relative potency factors of ER ligands vary between T47D-KBluc and ER-CALUX (Brand et al., 2013; Conley et al., 2016; Houtman et al., 2009; Sonneveld et al., 2005), the WFP treated water pre- and post- distribution likely do not contain levels of estrogenic compounds that would be predicted to cause adverse effects assuming standard rates of ingestion (WHO, 2000).
All estrogenic activity was detected in the intake water samples, but no environmental EBT has been developed for the T47D-KBluc. Escher et al. used environmental quality standards to derive EBT for several estrogen receptor agonist bioassays, which ranged from 0.1 (ER-CALUX) to 1.07 ng E2Eq/L (ISO-LYES) (Escher et al., 2018a). Given the variability in ecological EBTs, a conservative approach may be to use the predicted no effect concentration (PNEC) for long-term exposure to 17α-ethinyl estradiol (EE2; potent environmental ER agonist), 0.2 ng E2Eq/L (corrected for EE2 potency in the T47D-KBluc bioassay), presented by Caldwell et al (Caldwell et al., 2012; Conley et al., 2017a). Considering the Caldwell et al. PNEC, all samples with estrogenic activity above MDL contain levels likely to adversely affect aquatic species. A major contributor of the estrogenic activity detected in the Lake Michigan intake water samples is likely effluent from municipal wastewater treatment plants (WWTP) from the densely populated surrounding metropolitan area. WWTPs with standard activated sludge treatment may not be sufficient to remove the all estrogenic activity (Kibambe et al., 2020), however advanced wastewater treatment technologies including ozonation and sand filtration have been shown to completely remove all estrogenic activity from WWTP influent (Gehrmann et al., 2018).
We applied the CV1-chAR bioassay, originally created for screening environmental compounds (Hartig et al., 2002; Hartig et al., 2007), to water quality screening of each water sample extract. The CV1-chAR bioassay contains the chimpanzee androgen receptor (AR) that has 99.7% overall sequence similarity to the human AR ortholog and 100% sequence similarity in the ligand- and DNA-binding domains (Choong et al., 1998). The transcriptional activation bioassay was originally designed with the non-human primate receptor due to patent limitations on the use of the human AR (Hartig et al., 2007). We previously showed that the dihydrotestosterone-mediated transcriptional activation of both AR orthologs were statistically the same (Hartig et al., 2007).
Since the Chicago area water samples were concentrated during extraction and subsequently re-diluted 1000-fold in assay media we were able to seamlessly adapt the tool to environmental sample evaluation with no cytotoxicity issues. Of the 49 screened samples (including field and lab blanks), four produced androgenic activity (median: 0.93 ng DHTEq/L) and were comparable to androgenic activity previously detected in the US (4.7 ng DHTEq/L) (Conley et al., 2017a). The androgenic activity reported by Conley et al. was detected in US surface waters that were historically impacted by a variety of anthropogenic sources (e.g. industrial, municipal, agricultural). In studies aiming to optimize effects-directed analysis protocols, a limited number of surface water sites in the Netherlands (0.2 ng DHTEq/L) (Zwart et al., 2020), and several surface water sites across Europe (2.7 ng DHTEq/L) (Tousova et al., 2017) also resulted in androgenic activity comparable to activity detected in the Chicago pre-distribution samples.
Despite the 10 androgen receptor agonists included in the HPLC/MS-MS analysis (Table S7), no androgen analytes were detected above MDLs to account for the measured in vitro AR activity. Similar are results presented by Tousova et al. assessing European surface and wastewaters. AR activity ranged 0.93–2.7 DHTEqMDA-KB2/L, but AR agonists concentrations were negligible leaving the bioactivity entirely unexplained (Tousova et al., 2017). In our previous assessment of US impacted surface waters, up to 96% of the in vitro AR activity (MDA-KB2) was accounted for by measured testosterone, androstenedione, androsterone, and dihydrotestosterone environmental concentrations (Conley et al., 2017a).
There are many existing options for screening in vitro (anti)androgen activity (e.g. MDA-KB2, GeneBLAzer, CALUX, etc.). However, the CV1-chAR bioassay had a superior Z-factor (a simple statistic to evaluate the quality of high throughput screening assays) compared to androgenic activity detection methods we have used previously (Conley et al., 2017a; Medlock Kakaley et al., 2020). On a scale of 0–1, where 0 is an unacceptable assay and 1 is an ideal assay (Zhang et al., 1999), the Z-factor for the CV1-chAR is 0.76, while a comparable assay (which uses the same mechanism of action) we have previously used for water quality screening was 0.32 (MDA-KB2) (Conley et al., 2019).
Although no glucocorticoid activity was detected in the WFP intake, pre-, or post-distribution water samples, others have detected glucocorticoid receptor activity in wastewater effluent (Chang et al., 2007; Jia et al., 2016; Medlock Kakaley et al., 2020; Schriks et al., 2010; Suzuki et al., 2015; Tousova et al., 2017) and impacted surface waters (Tousova et al., 2017). Previously, we quantified glucocorticoid activity (above MDL) ranging 6.0–43 ng DexEqCV1-hGR/L in impacted surface and wastewater effluent specifically using the CV1-hGR bioassay (Conley et al., 2017a; Medlock Kakaley et al., 2020), although known glucocorticoid receptor ligands targeted in the complementary chemical analysis were not detected. In the comprehensive “budget balancing” exercise by Jia et al. triamcinolone acetonide, fluocinolone acetonide, clobetasol propionate, and fluticasone propionate were responsible for the majority of the detected GR activity (Jia et al., 2016). Discernibly all Chicago area samples fell below previously reported human health (drinking water) EBT of 21 (Brand et al., 2013) and 150 (Escher et al., 2015) ng DexEq/L, generated using the GR-CALUX bioassay.
5. Conclusions
We present a model that can expedite future testing of low activity samples (treated tapwater) without jeopardizing statistical stringency. Few detections of biological activity were identified in pre-distribution samples, but as we anticipated WFP treated post-distribution water samples did not produce any positive detections for endocrine activity above MDLs, and therefore likely do not contain any estrogenic, androgenic or glucocorticoid active compounds at concentrations that have the potential to cause adverse effects in humans.
Supplementary Material
Figure S1: Comparison of Cytotoxic Index Values of the USGS Water Samples S3
Figure S2: Flow diagram of the CHO cell chronic cytotoxicity assay S4
Table S1. Mammalian Bioassay Standard Compound EC50 Values
Table S2. Cytotoxicity Statistical Results and Cytotoxicity Index Values
Table S3. Yeast Bioassay Results
Table S4. Luminescent Mammalian Bioassay Results
Table S5. Steroid hormone information pertinent to analytical chemical detection performed by various laboratories for the U.S. Geological Survey Toxic Substances Hydrology Program
Table S6. Concentrations (μg/L) of target known steroid hormone analytes.
Highlights.
Corresponding pre- and post-distribution treated water samples were assessed
We applied a screening model for low level biological activity in water samples
Inaugural use of an (anti)androgenic bioassay for water quality screening
Estrogen and androgen activity were detected in untreated water
No biological activity detected in tapwater above method detection limits
Acknowledgements
The authors would like to thank Drs. Allison Camp and Erin Hines, and Celeste Journey for reviewing earlier manuscript drafts.
Funding
This work was supported by the USGS Environmental Health Mission Area; and the USEPA Safe and Sustainable Water Resources Program.
Footnotes
Competing Interests
The Authors have no competing interests to declare.
Disclaimer
The research described in this article has been reviewed by the Center for Public Health and Environmental Assessment within the Office of Research and Development, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views or policies of the Environmental Protection Agency, but do represent the views of the U.S. Geological Survey. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government. This report contains CAS Registry Numbers®, which is a Registered Trademark of the American Chemical Society. CAS recommends the verification of the CASRNs through CAS Client ServicesSM.
References
- Bhatia SK, & Yetter AB (2008). Correlation of visual in vitro cytotoxicity ratings of biomaterials with quantitative in vitro cell viability measurements. Cell Biol Toxicol, 24(4), 315–319. doi: 10.1007/s10565-007-9040-z [DOI] [PubMed] [Google Scholar]
- Blackwell BR, Ankley GT, Bradley PM, Houck KA, Makarov SS, Medvedev AV, Swintek J, & Villeneuve DL (2019). Potential Toxicity of Complex Mixtures in Surface Waters from a Nationwide Survey of United States Streams: Identifying in Vitro Bioactivities and Causative Chemicals. Environmental Science & Technology, 53(2), 973–983. doi: 10.1021/acs.est.8b05304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell BR, Ankley GT, Corsi SR, DeCicco LA, Houck KA, Judson RS, Li S, Martin MT, Murphy E, Schroeder AL, Smith ER, Swintek J, & Villeneuve DL (2017). An “EAR” on Environmental Surveillance and Monitoring: A Case Study on the Use of Exposure–Activity Ratios (EARs) to Prioritize Sites, Chemicals, and Bioactivities of Concern in Great Lakes Waters. Environmental Science & Technology, 51(15), 8713–8724. doi: 10.1021/acs.est.7b01613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GEP, Hunter WG, & S HJ (1978). Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. New York, NY: Wiley & Sons Inc. [Google Scholar]
- Brack W, Altenburger R, Schüürmann G, Krauss M, López Herráez D, van Gils J, Slobodnik J, Munthe J, Gawlik BM, van Wezel A, Schriks M, Hollender J, Tollefsen KE, Mekenyan O, Dimitrov S, Bunke D, Cousins I, Posthuma L, van den Brink PJ, López de Alda M, Barceló D, Faust M, Kortenkamp A, Scrimshaw M, Ignatova S, Engelen G, Massmann G, Lemkine G, Teodorovic I, Walz K-H, Dulio V, Jonker MTO, Jäger F, Chipman K, Falciani F, Liska I, Rooke D, Zhang X, Hollert H, Vrana B, Hilscherova K, Kramer K, Neumann S, Hammerbacher R, Backhaus T, Mack J, Segner H, Escher B, & de Aragão Umbuzeiro G. (2015). The SOLUTIONS project: Challenges and responses for present and future emerging pollutants in land and water resources management. Science of The Total Environment, 503–504, 22–31. doi: 10.1016/j.scitotenv.2014.05.143 [DOI] [PubMed] [Google Scholar]
- Bradley PM, Argos M, Kolpin DW, Meppelink SM, Romanok KM, Smalling KL, Focazio MJ, Allen JM, Dietze JE, Devito MJ, Donovan AR, Evans N, Givens CE, Gray JL, Higgins CP, Hladik ML, Iwanowicz LR, Journey CA, Lane RF, Laughrey ZR, Loftin KA, McCleskey RB, McDonough CA, Medlock-Kakaley E, Meyer MT, Putz AR, Richardson SD, Stark AE, Weis CP, Wilson VS, & Zehraoui A. (2020). Mixed organic and inorganic tapwater exposures and potential effects in greater Chicago area, USA. Science of The Total Environment, 719, 137236. doi: 10.1016/j.scitotenv.2020.137236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Journey CA, Romanok KM, Barber LB, Buxton HT, Foreman WT, Furlong ET, Glassmeyer ST, Hladik ML, Iwanowicz LR, Jones DK, Kolpin DW, Kuivila KM, Loftin KA, Mills MA, Meyer MT, Orlando JL, Reilly TJ, Smalling KL, & Villeneuve DL (2017). Expanded Target-Chemical Analysis Reveals Extensive Mixed-Organic-Contaminant Exposure in U.S. Streams. Environmental Science & Technology, 51(9), 4792–4802. doi: 10.1021/acs.est.7b00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PM, Kolpin DW, Romanok KM, Smalling KL, Focazio MJ, Brown JB, Cardon MC, Carpenter KD, Corsi SR, DeCicco LA, Dietze JE, Evans N, Furlong ET, Givens CE, Gray JL, Griffin DW, Higgins CP, Hladik ML, Iwanowicz LR, Journey CA, Kuivila KM, Masoner JR, McDonough CA, Meyer MT, Orlando JL, Strynar MJ, Weis CP, & Wilson VS (2018). Reconnaissance of Mixed Organic and Inorganic Chemicals in Private and Public Supply Tapwaters at Selected Residential and Workplace Sites in the United States. Environmental Science & Technology, 52(23), 13972–13985. doi: 10.1021/acs.est.8b04622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand W, de Jongh CM, van der Linden SC, Mennes W, Puijker LM, van Leeuwen CJ, van Wezel AP, Schriks M, & Heringa MB (2013). Trigger values for investigation of hormonal activity in drinking water and its sources using CALUX bioassays. Environment International, 55, 109–118. doi: 10.1016/j.envint.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Caldwell DJ, Mastrocco F, Anderson PD, Länge R, & Sumpter JP (2012). Predicted-no-effect concentrations for the steroid estrogens estrone, 17β-estradiol, estriol, and 17α-ethinylestradiol. Environmental Toxicology and Chemistry, 31(6), 1396–1406. doi: 10.1002/etc.1825 [DOI] [PubMed] [Google Scholar]
- Chang H, Hu J, & Shao B. (2007). Occurrence of Natural and Synthetic Glucocorticoids in Sewage Treatment Plants and Receiving River Waters. Environmental Science & Technology, 41(10), 3462–3468. doi: 10.1021/es062746o [DOI] [PubMed] [Google Scholar]
- Choong CS, Kemppainen JA, & Wilson EM (1998). Evolution of the Primate Androgen Receptor: A Structural Basis for Disease. Journal of Molecular Evolution, 47(3), 334–342. doi: 10.1007/PL00006391 [DOI] [PubMed] [Google Scholar]
- Ciparis S, Iwanowicz LR, & Voshell JR (2012). Effects of watershed densities of animal feeding operations on nutrient concentrations and estrogenic activity in agricultural streams. Science of The Total Environment, 414, 268–276. doi: 10.1016/j.scitotenv.2011.10.017 [DOI] [PubMed] [Google Scholar]
- Conley JM, Evans N, Cardon MC, Medlock Kakaley E, Hartig PC, Gray LE Jr, & Wilson VS (2019). In vitro hormone receptor transcriptional activation assays for screening of water samples. Overview of Bioasay Research. USEPA. External Agency Communications. [Google Scholar]
- Conley JM, Evans N, Cardon MC, Rosenblum L, Iwanowicz LR, Hartig PC, Schenck KM, Bradley PM, & Wilson VS (2017a). Occurrence and In Vitro Bioactivity of Estrogen, Androgen, and Glucocorticoid Compounds in a Nationwide Screen of United States Stream Waters. Environmental Science & Technology, 51(9), 4781–4791. doi: 10.1021/acs.est.6b06515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Evans N, Mash H, Rosenblum L, Schenck K, Glassmeyer S, Furlong ET, Kolpin DW, & Wilson VS (2017b). Comparison of in vitro estrogenic activity and estrogen concentrations in source and treated waters from 25 U.S. drinking water treatment plants. Science of The Total Environment, 579(Supplement C), 1610–1617. doi: 10.1016/j.scitotenv.2016.02.093 [DOI] [PubMed] [Google Scholar]
- Conley JM, Hannas BR, Furr JR, Wilson VS, & Gray LE (2016). A Demonstration of the Uncertainty in Predicting the Estrogenic Activity of Individual Chemicals and Mixtures From an In Vitro Estrogen Receptor Transcriptional Activation Assay (T47D-KBluc) to the In Vivo Uterotrophic Assay Using Oral Exposure. Toxicological Sciences, 153(2), 382–395. doi: 10.1093/toxsci/kfw134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler D, & Miller G. (2005). The role of public health improvements in health advances: The twentieth-century United States. Demography, 42(1), 1–22. doi: 10.1353/dem.2005.0002 [DOI] [PubMed] [Google Scholar]
- Daiber EJ, DeMarini DM, Ravuri SA, Liberatore HK, Cuthbertson AA, Thompson-Klemish A, Byer JD, Schmid JE, Afifi MZ, Blatchley ER, & Richardson SD (2016). Progressive Increase in Disinfection Byproducts and Mutagenicity from Source to Tap to Swimming Pool and Spa Water: Impact of Human Inputs. Environmental Science & Technology, 50(13), 6652–6662. doi: 10.1021/acs.est.6b00808 [DOI] [PubMed] [Google Scholar]
- Denison MS, Mehinto AC, Olivieri A, Plummlee M, Schlenk D, Thompson S, & Waggoner C. (2020). Bioanalytical Tools for Detection and Quantification of Estrogenic and Dioxin-Like Chemicals in Water Recycling and Reuse: Guidance Document for Developing a Standard Operating Procedure. National Water Research Institute. [Google Scholar]
- Dobson CM (2004). Chemical space and biology. Nature, 432(7019), 824–828. doi: 10.1038/nature03192 [DOI] [PubMed] [Google Scholar]
- Dong H, Cuthbertson AA, & Richardson SD (2020). Effect-Directed Analysis (EDA): A Promising Tool for Nontarget Identification of Unknown Disinfection Byproducts in Drinking Water. Environmental Science & Technology, 54(3), 1290–1292. doi: 10.1021/acs.est.0c00014 [DOI] [PubMed] [Google Scholar]
- Dong S, Masalha N, Plewa MJ, & Nguyen TH (2017). Toxicity of Wastewater with Elevated Bromide and Iodide after Chlorination, Chloramination, or Ozonation Disinfection. Environmental Science & Technology, 51(16), 9297–9304. doi: 10.1021/acs.est.7b02345 [DOI] [PubMed] [Google Scholar]
- Dong S, Page MA, Massalha N, Hur A, Hur K, Bokenkamp K, Wagner ED, & Plewa MJ (2019). Toxicological Comparison of Water, Wastewaters, and Processed Wastewaters. Environmental Science & Technology, 53(15), 9139–9147. doi: 10.1021/acs.est.9b00827 [DOI] [PubMed] [Google Scholar]
- Dusza HM, Janssen E, Kanda R, & Legler J. (2019). Method Development for Effect-Directed Analysis of Endocrine Disrupting Compounds in Human Amniotic Fluid. Environmental Science & Technology, 53(24), 14649–14659. doi: 10.1021/acs.est.9b04255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher BI, Allinson M, Altenburger R, Bain PA, Balaguer P, Busch W, Crago J, Denslow ND, Dopp E, Hilscherova K, Humpage AR, Kumar A, Grimaldi M, Jayasinghe BS, Jarosova B, Jia A, Makarov S, Maruya KA, Medvedev A, Mehinto AC, Mendez JE, Poulsen A, Prochazka E, Richard J, Schifferli A, Schlenk D, Scholz S, Shiraishi F, Snyder S, Su G, Tang JYM, Burg B. v. d., Linden S. C. v. d., Werner I, Westerheide SD, Wong CKC, Yang M, Yeung BHY, Zhang X, & Leusch FDL (2014). Benchmarking Organic Micropollutants in Wastewater, Recycled Water and Drinking Water with In Vitro Bioassays. Environmental Science & Technology, 48(3), 1940–1956. doi: 10.1021/es403899t [DOI] [PubMed] [Google Scholar]
- Escher BI, Aїt-Aїssa S, Behnisch PA, Brack W, Brion F, Brouwer A, Buchinger S, Crawford SE, Du Pasquier D, Hamers T, Hettwer K, Hilscherová K, Hollert H, Kase R, Kienle C, Tindall AJ, Tuerk J, van der Oost R, Vermeirssen E, & Neale PA (2018a). Effect-based trigger values for in vitro and in vivo bioassays performed on surface water extracts supporting the environmental quality standards (EQS) of the European Water Framework Directive. Science of The Total Environment, 628–629, 748–765. doi: 10.1016/j.scitotenv.2018.01.340 [DOI] [PubMed] [Google Scholar]
- Escher BI, Neale PA, & Leusch FDL (2015). Effect-based trigger values for in vitro bioassays: Reading across from existing water quality guideline values. Water Research, 81, 137–148. doi: 10.1016/j.watres.2015.05.049 [DOI] [PubMed] [Google Scholar]
- Escher BI, Neale PA, & Villeneuve DL (2018b). The advantages of linear concentration–response curves for in vitro bioassays with environmental samples. Environmental Toxicology and Chemistry, 37(9), 2273–2280. doi: 10.1002/etc.4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann L, Bielak H, Behr M, Itzel F, Lyko S, Simon A, Kunze G, Dopp E, Wagner M, & Tuerk J. (2018). (Anti-)estrogenic and (anti-)androgenic effects in wastewater during advanced treatment: comparison of three in vitro bioassays. Environmental Science and Pollution Research, 25(5), 4094–4104. doi: 10.1007/s11356-016-7165-4 [DOI] [PubMed] [Google Scholar]
- Hartig PC, Bobseine KL, Britt BH, Cardon MC, Lambright CR, Wilson VS, & Gray LE Jr. (2002). Development of two androgen receptor assays using adenoviral transduction of MMTV-luc reporter and/or hAR for endocrine screening. Toxicol Sci, 66(1), 82–90. [DOI] [PubMed] [Google Scholar]
- Hartig PC, Cardon MC, Lambright CR, Bobseine KL, Gray LE Jr, & Wilson VS (2007). Substitution of synthetic chimpanzee androgen receptor for human androgen receptor in competitive binding and transcriptional activation assays for EDC screening. Toxicology Letters, 174(1–3), 89–97. doi: 10.1016/j.toxlet.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Hashmi MAK, Krauss M, Escher BI, Teodorovic I, & Brack W. (2020). Effect-Directed Analysis of Progestogens and Glucocorticoids at Trace Concentrations in River Water. Environmental Toxicology and Chemistry, 39(1), 189–199. doi: 10.1002/etc.4609 [DOI] [PubMed] [Google Scholar]
- Houtman CJ, Sterk SS, van de Heijning MPM, Brouwer A, Stephany RW, van der Burg B, & Sonneveld E. (2009). Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays. Analytica Chimica Acta, 637(1), 247–258. doi: 10.1016/j.aca.2008.09.037 [DOI] [PubMed] [Google Scholar]
- Hyde T. (2015). Final Report: High Lead at Three Residences in Flint, Michigan. Chicago, IL: USEPA. [Google Scholar]
- Jeong CH, Wagner ED, Siebert VR, Anduri S, Richardson SD, Daiber EJ, McKague AB, Kogevinas M, Villanueva CM, Goslan EH, Luo W, Isabelle LM, Pankow JF, Grazuleviciene R, Cordier S, Edwards SC, Righi E, Nieuwenhuijsen MJ, & Plewa MJ (2012). Occurrence and Toxicity of Disinfection Byproducts in European Drinking Waters in Relation with the HIWATE Epidemiology Study. Environmental Science & Technology, 46(21), 12120–12128. doi: 10.1021/es3024226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia A, Escher BI, Leusch FDL, Tang JYM, Prochazka E, Dong B, Snyder EM, & Snyder SA (2015). In vitro bioassays to evaluate complex chemical mixtures in recycled water. Water Research, 80, 1–11. doi: 10.1016/j.watres.2015.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia A, Wu S, Daniels KD, & Snyder SA (2016). Balancing the Budget: Accounting for Glucocorticoid Bioactivity and Fate during Water Treatment. Environmental Science & Technology, 50(6), 2870–2880. doi: 10.1021/acs.est.5b04893 [DOI] [PubMed] [Google Scholar]
- K S, & M X. (2008). Bootstrap: A Statistical Method. Rutgers University; Newbrunswick, NJ. [Google Scholar]
- Kassotis CD, Iwanowicz LR, Akob DM, Cozzarelli IM, Mumford AC, Orem WH, & Nagel SC (2016). Endocrine disrupting activities of surface water associated with a West Virginia oil and gas industry wastewater disposal site. Science of The Total Environment, 557–558, 901–910. doi: 10.1016/j.scitotenv.2016.03.113 [DOI] [PubMed] [Google Scholar]
- Kibambe MG, Momba MNB, Daso AP, Van Zijl MC, & Coetzee MAA (2020). Efficiency of selected wastewater treatment processes in removing estrogen compounds and reducing estrogenic activity using the T47D-KBLUC reporter gene assay. Journal of Environmental Management, 260, 110135. doi: 10.1016/j.jenvman.2020.110135 [DOI] [PubMed] [Google Scholar]
- Könemann S, Kase R, Simon E, Swart K, Buchinger S, Schlüsener M, Hollert H, Escher BI, Werner I, Aït-Aïssa S, Vermeirssen E, Dulio V, Valsecchi S, Polesello S, Behnisch P, Javurkova B, Perceval O, Di Paolo C, Olbrich D, Sychrova E, Schlichting R, Leborgne L, Clara M, Scheffknecht C, Marneffe Y, Chalon C, Tušil P, Soldàn P, von Danwitz B, Schwaiger J, San Martín Becares MI, Bersani F, Hilscherová K, Reifferscheid G, Ternes T, & Carere M. (2018). Effect-based and chemical analytical methods to monitor estrogens under the European Water Framework Directive. TrAC Trends in Analytical Chemistry, 102, 225–235. doi: 10.1016/j.trac.2018.02.008 [DOI] [Google Scholar]
- Leusch FDL, Neale PA, Arnal C, Aneck-Hahn NH, Balaguer P, Bruchet A, Escher BI, Esperanza M, Grimaldi M, Leroy G, Scheurer M, Schlichting R, Schriks M, & Hebert A. (2018). Analysis of endocrine activity in drinking water, surface water and treated wastewater from six countries. Water Research, 139, 10–18. doi: 10.1016/j.watres.2018.03.056 [DOI] [PubMed] [Google Scholar]
- Martin JJ, Winslow SD, & Munch DJ (2007). A New Approach to Drinking-Water-Quality Data: Lowest-Concentration Minimum Reporting Level. Environmental Science & Technology, 41(3), 677–681. doi: 10.1021/es072456n [DOI] [PubMed] [Google Scholar]
- Medlock Kakaley E, Cardon MC, Gray LE, Hartig PC, & Wilson VS (2018). Generalized Concentration Addition Model Predicts Glucocorticoid Activity Bioassay Responses to Environmentally Detected Receptor-Ligand Mixtures. Toxicological Sciences, 168(1), 252–263. doi: 10.1093/toxsci/kfy290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock Kakaley EK, Blackwell BR, Cardon MC, Conley JM, Evans N, Feifarek DJ, Furlong ET, Glassmeyer ST, Gray LE, Hartig PC, Kolpin DW, Mills MA, Rosenblum L, Villeneuve DL, & Wilson VS (2020). De Facto Water Reuse: Bioassay suite approach delivers depth and breadth in endocrine active compound detection. Science of The Total Environment, 699, 134297. doi: 10.1016/j.scitotenv.2019.134297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CC, Vatankhah H, McDonough CA, Nickerson A, Hedtke TT, Cath TY, Higgins CP, & Bellona CL (2019). Removal of per- and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. Journal of Hazardous Materials, 366, 160–168. doi: 10.1016/j.jhazmat.2018.11.050 [DOI] [PubMed] [Google Scholar]
- Neale PA, Ait-Aissa S, Brack W, Creusot N, Denison MS, Deutschmann B, Hilscherová K, Hollert H, Krauss M, Novák J, Schulze T, Seiler T-B, Serra H, Shao Y, & Escher BI (2015). Linking in Vitro Effects and Detected Organic Micropollutants in Surface Water Using Mixture-Toxicity Modeling. Environmental Science & Technology, 49(24), 14614–14624. doi: 10.1021/acs.est.5b04083 [DOI] [PubMed] [Google Scholar]
- Plewa M, & Wagner E. (2015). Charting a New Path To Resolve the Adverse Health Effects of DBPs (pp. 3–23). [Google Scholar]
- Plewa MJ, Kargalioglu Y, Vankerk D, Minear RA, & Wagner ED (2002). Mammalian cell cytotoxicity and genotoxicity analysis of drinking water disinfection by-products. Environmental and Molecular Mutagenesis, 40(2), 134–142. doi: 10.1002/em.10092 [DOI] [PubMed] [Google Scholar]
- Plewa MJ, Simmons JE, Richardson SD, & Wagner ED (2010). Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products. Environmental and Molecular Mutagenesis, 51(8‐9), 871–878. doi: 10.1002/em.20585 [DOI] [PubMed] [Google Scholar]
- Romanok KM, Kolpin DW, Meppelink SM, Argos M, Brown JB, Devito MJ, Dietze JE, Givens CE, Gray JL, Higgins CP, Hladik ML, Iwanowicz LR, Loftin KA, McCleskey RB, McDonough CA, Meyer MT, Strynar MJ, Weis CP, Wilson VS, & Bradley PM (2018). Methods used for the collection and analysis of chemical and biological data for the Tapwater Exposure Study, United States, 2016–17 Open-File Report. Reston, VA. [Google Scholar]
- Sanseverino J, Gupta RK, Layton AC, Patterson SS, Ripp SA, Saidak L, Simpson ML, Schultz TW, & Sayler GS (2005). Use of <em>Saccharomyces cerevisiae</em> BLYES Expressing Bacterial Bioluminescence for Rapid, Sensitive Detection of Estrogenic Compounds. Applied and Environmental Microbiology, 71(8), 4455. doi: 10.1128/AEM.71.8.4455-4460.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenen D. (2002). Role of disinfection in suppressing the spread of pathogens with drinking water: possibilities and limitations. Water Research, 36(15), 3874–3888. doi: 10.1016/S0043-1354(02)00076-3 [DOI] [PubMed] [Google Scholar]
- Schriks M, van Leerdam JA, van der Linden SC, van der Burg B, van Wezel AP, & de Voogt P. (2010). High-Resolution Mass Spectrometric Identification and Quantification of Glucocorticoid Compounds in Various Wastewaters in The Netherlands. Environmental Science & Technology, 44(12), 4766–4774. doi: 10.1021/es100013x [DOI] [PubMed] [Google Scholar]
- Shi P, Zhou S, Xiao H, Qiu J, Li A, Zhou Q, Pan Y, & Hollert H. (2018). Toxicological and chemical insights into representative source and drinking water in eastern China. Environmental Pollution, 233, 35–44. doi: 10.1016/j.envpol.2017.10.033 [DOI] [PubMed] [Google Scholar]
- Snyder SA, & Leusch FDL (2018). State of the Science Report In vItro bioassays: Current status and future application for water manangement: Global Water Research Coalition. [Google Scholar]
- Sonneveld E, Riteco JAC, Jansen HJ, Pieterse B, Brouwer A, Schoonen WG, & van der Burg B. (2005). Comparison of In Vitro and In Vivo Screening Models for Androgenic and Estrogenic Activities. Toxicological Sciences, 89(1), 173–187. doi: 10.1093/toxsci/kfj009 [DOI] [PubMed] [Google Scholar]
- Suzuki G, Sato K, Isobe T, Takigami H, Brouwer A, & Nakayama K. (2015). Detection of glucocorticoid receptor agonists in effluents from sewage treatment plants in Japan. Science of The Total Environment, 527–528, 328–334. doi: 10.1016/j.scitotenv.2015.05.008 [DOI] [PubMed] [Google Scholar]
- Tousova Z, Oswald P, Slobodnik J, Blaha L, Muz M, Hu M, Brack W, Krauss M, Di Paolo C, Tarcai Z, Seiler T-B, Hollert H, Koprivica S, Ahel M, Schollée JE, Hollender J, Suter MJF, Hidasi AO, Schirmer K, Sonavane M, Ait-Aissa S, Creusot N, Brion F, Froment J, Almeida AC, Thomas K, Tollefsen KE, Tufi S, Ouyang X, Leonards P, Lamoree M, Torrens VO, Kolkman A, Schriks M, Spirhanzlova P, Tindall A, & Schulze T. (2017). European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters. Science of The Total Environment, 601–602, 1849–1868. doi: 10.1016/j.scitotenv.2017.06.032 [DOI] [PubMed] [Google Scholar]
- UNWAPP. (2017). The United Nations World Water Development Report. Paris: UNESCO. [Google Scholar]
- van der Oost R, Sileno G, Janse T, Nguyen MT, Besselink H, & Brouwer A. (2017). SIMONI (Smart Integrated Monitoring) as a novel bioanalytical strategy for water quality assessment: Part II–field feasibility survey. Environmental Toxicology and Chemistry, 36(9), 2400–2416. doi: 10.1002/etc.3837 [DOI] [PubMed] [Google Scholar]
- Vasquez MI, Lambrianides A, Schneider M, Kümmerer K, & Fatta-Kassinos D. (2014). Environmental side effects of pharmaceutical cocktails: What we know and what we should know. Journal of Hazardous Materials, 279, 169–189. doi: 10.1016/j.jhazmat.2014.06.069 [DOI] [PubMed] [Google Scholar]
- Wagner ED, & Plewa MJ (2017). CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: An updated review. Journal of Environmental Sciences, 58, 64–76. doi: 10.1016/j.jes.2017.04.021 [DOI] [PubMed] [Google Scholar]
- WHO. (2000). Food Additives Series: 43 Toxicological Evaluation of Certain Veterinary Drug Residues in Food. [Google Scholar]
- Wilson VS, Bobseine K, & Gray JLE (2004). Development and Characterization of a Cell Line That Stably Expresses an Estrogen-Responsive Luciferase Reporter for the Detection of Estrogen Receptor Agonist and Antagonists. Toxicological Sciences, 81(1), 69–77. doi: 10.1093/toxsci/kfh180 [DOI] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Lambright CR, & Gray JLE (2002). A Novel Cell Line, MDA-kb2, That Stably Expresses an Androgen- and Glucocorticoid-Responsive Reporter for the Detection of Hormone Receptor Agonists and Antagonists. Toxicological Sciences, 66(1), 69–81. doi: 10.1093/toxsci/66.1.69 [DOI] [PubMed] [Google Scholar]
- Yost EE, Meyer MT, Dietze JE, Meissner BM, Worley-Davis L, Williams CM, Lee B, & Kullman SW (2013). Comprehensive Assessment of Hormones, Phytoestrogens, and Estrogenic Activity in an Anaerobic Swine Waste Lagoon. Environmental Science & Technology, 47(23), 13781–13790. doi: 10.1021/es4026408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost EE, Meyer MT, Dietze JE, Williams CM, Worley-Davis L, Lee B, & Kullman SW (2014). Transport of Steroid Hormones, Phytoestrogens, and Estrogenic Activity across a Swine Lagoon/Sprayfield System. Environmental Science & Technology, 48(19), 11600–11609. doi: 10.1021/es5025806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-H, Chung TDY, & Oldenburg KR (1999). A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of Biomolecular Screening, 4(2), 67–73. doi: 10.1177/108705719900400206 [DOI] [PubMed] [Google Scholar]
- Zhen H, Ekman DR, Collette TW, Glassmeyer ST, Mills MA, Furlong ET, Kolpin DW, & Teng Q. (2018). Assessing the impact of wastewater treatment plant effluent on downstream drinking water-source quality using a zebrafish (Danio Rerio) liver cell-based metabolomics approach. Water Research, 145, 198–209. doi: 10.1016/j.watres.2018.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart N, Jonker W, Broek R. t., de Boer J, Somsen G, Kool J, Hamers T, Houtman CJ, & Lamoree MH (2020). Identification of mutagenic and endocrine disrupting compounds in surface water and wastewater treatment plant effluents using high-resolution effect-directed analysis. Water Research, 168, 115204. doi: 10.1016/j.watres.2019.115204 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Comparison of Cytotoxic Index Values of the USGS Water Samples S3
Figure S2: Flow diagram of the CHO cell chronic cytotoxicity assay S4
Table S1. Mammalian Bioassay Standard Compound EC50 Values
Table S2. Cytotoxicity Statistical Results and Cytotoxicity Index Values
Table S3. Yeast Bioassay Results
Table S4. Luminescent Mammalian Bioassay Results
Table S5. Steroid hormone information pertinent to analytical chemical detection performed by various laboratories for the U.S. Geological Survey Toxic Substances Hydrology Program
Table S6. Concentrations (μg/L) of target known steroid hormone analytes.