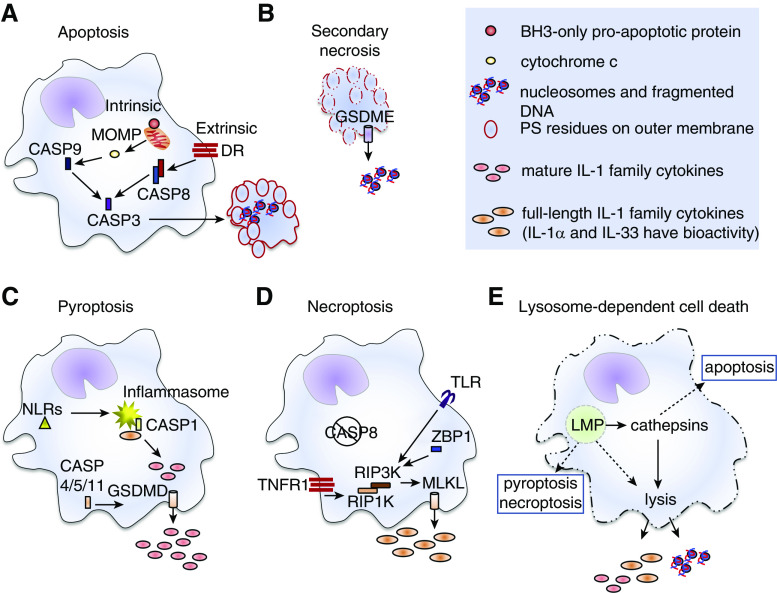
Figure 1.
Pulmonary macrophage regulated-cell-death modalities. (A) Intrinsic apoptosis is induced by intracellular or extracellular alterations to a cell’s microenvironment that lead to activation of BH3 (Bcl2 homology domain 3)–only proapoptotic proteins and mitochondrial outer-membrane permeabilization (MOMP). Release of cytochrome c drives activation of CASP9 (caspase 9). Extrinsic apoptosis is induced by receptors on the plasma membranes of cells, including death receptors (DR) in the TNF superfamily. Engagement of these receptors promotes activation of CASP8. Active CASP8 and/or CASP9 activate the executioner CASP3 enzyme that initiates the apoptotic program, leading to DNA fragmentation, flipping of phosphatidylserine (PS) residues to the outer leaflet of the plasma membrane (red outline), breakdown of the nuclear membrane, and packaging of cellular contents into apoptotic bodies. Apoptotic cells and bodies are either cleared by other phagocytes via efferocytosis and die within lysosomes (not shown) or (B) undergo secondary necrosis in which the plasma membranes fall apart (dashed red outline) and damage-associated molecular patterns (DAMPs) are released. Secondary necrosis has been associated with nucleosome release, and may involve CASP3-mediated activation of GSDME (gasdermin E), which forms pore-like structures in the plasma membrane (cylinder with arrow). (C) Pyroptosis results after activation of NLRs (Nod-like receptors, yellow triangle) that promote assembly of the inflammasome (yellow starburst) and activation of CASP1. Other inflammatory caspases (CASP4, CASP5 and/or CASP11) can be activated by intracellular pathogens independently of the inflammasome. In macrophages that express full-length precursor forms of certain members of the IL-1 family, active CASP1 cleaves them into the mature cytokines. This enhances their biological activity and is essential for IL-1β and IL-18 bioactivity. The active forms of proinflammatory caspase enzymes also promote activation of GSDMD (gasdermin D), which forms pore-like structures (cylinder with arrow) in the plasma membrane. Mature IL-1 family cytokines are released through these pores and plasma membrane integrity (solid cell outlines) is weakened. (D) Necroptosis can be induced as an alternative cell-death pathway when apoptotic CASP8 activity is impaired (negation symbol). It can be induced by TLR (Toll-like receptor), ZBP1 (Z conformation DNA [Z-DNA]-binding protein 1), or TNFR1 (TNF receptor 1) signaling events that activate RIP3K (receptor-interacting serine/threonine-protein kinase 3), an enzyme that cleaves mixed-lineage kinase domain–like pseudokinase (MLKL). Cleaved MLKL proteins self-oligomerize and form pore-like structures in the plasma membrane (cylinder with arrow) and thus promote release of small DAMPs, like IL-1 family members, and promote influx of ions into the cells leading to disruption of the plasma membrane. (E) Lysosome-dependent cell death occurs after lysosome membrane permeabilization (LMP). A defining feature of lysosome-dependent cell death is the release of cathepsin enzymes into the cytosol that promote (solid arrows) catastrophic rupture of the cells (dashed cell outline). Depending upon the degree of LMP, other overlapping cell death pathways or cathepsin-independent cell lysis (dashed arrows) can be induced. RIP1K = receptor-interacting serine/threonine-protein kinase 1.