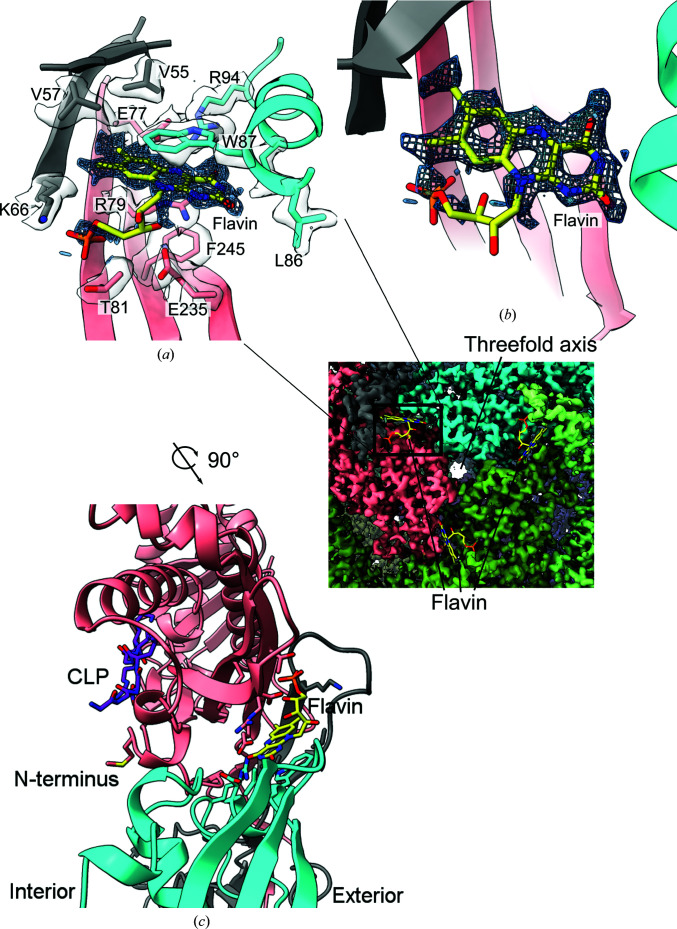
Figure 3.
(a) Density for a tricyclic ligand was found in the cryo-EM map, consistent with flavin mononucleotide (FMN). Amino acids within 5 Å that form the binding pocket are labeled. Only density for FMN and the amino-acid side chains is shown for clarity. Different colors correspond to different subunits. The inset shows three flavin-binding sites on the exterior of TmEnc around the threefold axis. (b) An overhead view of the flavin density and the modeled FMN with the surrounding amino acids hidden. (c) The view from (a) is rotated 90° clockwise along the z axis pointing out of the page and shows that the flavin-binding pocket on the exterior is on the opposite side to the cargo-loading peptide (CLP) and N-terminus in the interior of TmEnc. The cargo protein is not present in the cryo-EM structure, so the CLP from the crystal structure was aligned and placed in the cryo-EM structure. The distance between the centroids of the FMN and CLP is 21.4 Å, which is slightly less than the thickness of the TmEnc compartment.