Fig. 2.
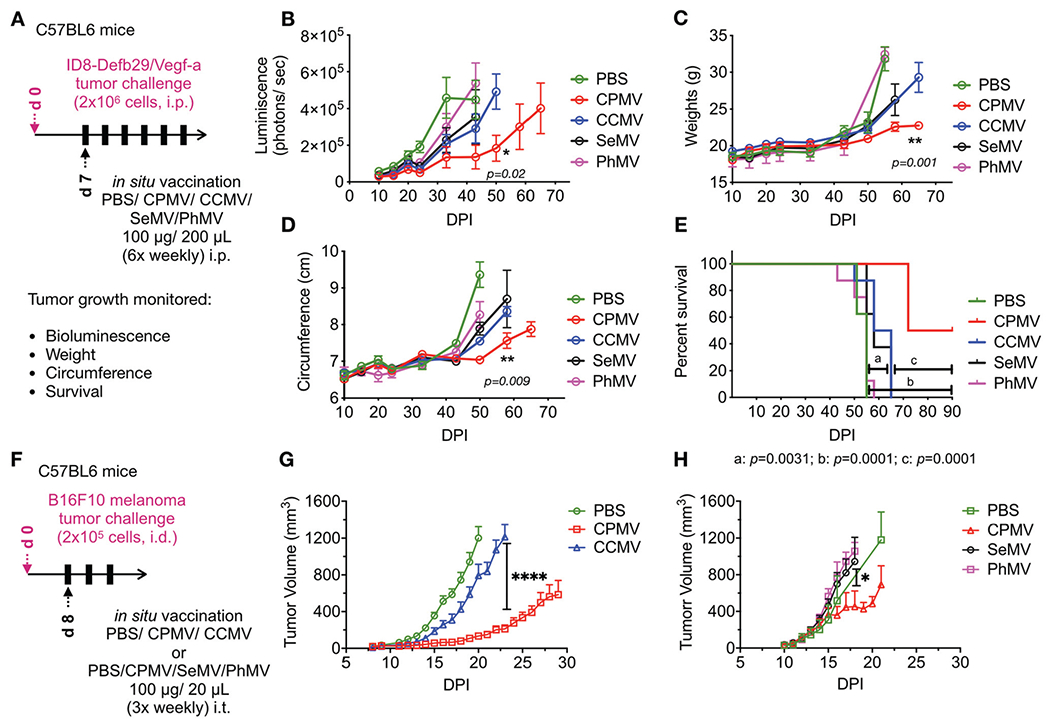
CPMV in situ vaccine has higher therapeutic efficacy over other plant viruses in mouse models of ID8-Defb29/Vegf-a ovarian cancer and B16F10 dermal melanoma. (A) Female C57BL6 mice were inoculated with 2 × 106 luciferase expressing ID8-Defb29/Vegf-a ovarian cancer cells intra-peritoneally. CPMV, CCMV, SeMV and PhMV were administered i.p. 6× weekly (100 μg/200 μL PBS) starting 7 days post-tumor inoculation. Tumor progression was monitored by bioluminescence imaging (B), and by measuring gains in weights (C) and circumference (D) from increasing tumor burden and developing ascites, as well as overall survival (E). Results were compared using unpaired t-test in B-D (with *=p < 0.05, **=p < 0.01) and Log-Rank Mantel-Cox test on survival data (****=p < 0.0001, ***=p < 0.001, **=p < 0.01). (F) C57BL6 mice were challenged with dermal B16F10 tumors by injecting 2 × 105 cells intradermally and treated with 3× weekly intratumoral injections of PBS vs. CPMV vs. CCMV (G) or PBS vs. CPMV vs. SeMV vs. PhMV (H) starting on day 8 from tumor challenge. Treatment efficacy was measured by monitoring tumor volumes. Results were compared using unpaired t-test in G (with ****=p < 0.0001) and one-way ANOVA in H (with *=p < 0.05).
