Figure 2.
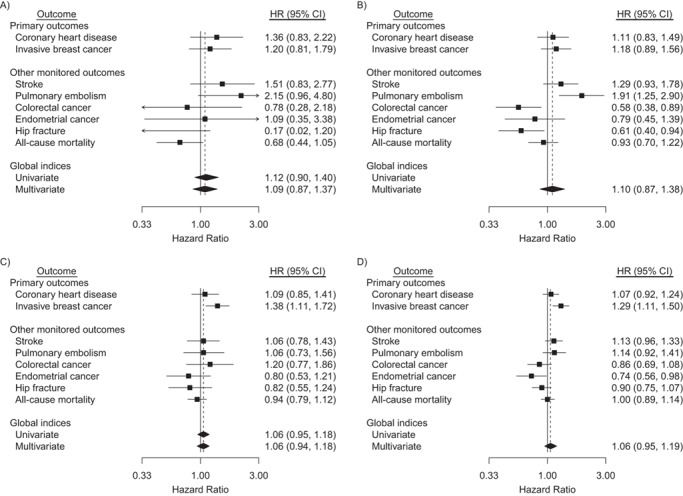
Hazard ratios (HRs) and 95% confidence intervals (CIs) for outcomes in the conjugated equine estrogens (CEE) + medroxyprogesterone acetate (MPA) trial during the intervention phase and cumulative follow-up among participants aged 50–59 years at randomization, Women’s Health Initiative, United States, 1993–2016. Participants were postmenopausal and aged 50–79 when enrolled at 40 US clinical centers during 1993–1998. Cumulative follow-up is through 2016. Panels A and B summarize the intervention phase, and panels C and D summarize cumulative follow-up. HRs for primary and other monitored outcomes are from a multivariate marginal Cox regression model stratified by 10-year age group, randomization status in the Diet Modification trial, prior disease (if applicable), race/ethnicity, prior hormone therapy use, study phase (time-dependent), and outcome type. The left forest plots (saturated model, panels A and C) are derived by including regression terms for CEE + MPA and product interaction terms for CEE + MPA × age group, CEE + MPA × outcome type, and CEE + MPA × outcome type × age group. The right forest plots (parsimonious model, panels B and D) also provide estimated HRs for women aged 50–59 years but drop the 3-factor interaction, CEE + MPA × age group × outcome type, so that estimates are based on a common CEE + MPA × outcome type interaction. Dashed reference lines correspond to estimated HR for multivariate global indices. Similar summaries for women aged 60–69 and 70–79 years can be found in supplemental Web Figures 3a and 3b, respectively. Time to first event of any monitored outcome defines the univariate global index; summary statistics are from a univariate Cox regression model with stratification described above. Marginal estimate of a common HR for monitored outcomes defines the multivariate global index. Robust sandwich-estimators for variances account within participant correlation of multivariate failure times. For consistency with other monitored outcomes included in the global index, extended follow-up includes only participants who provided consent for long-term follow-up.
