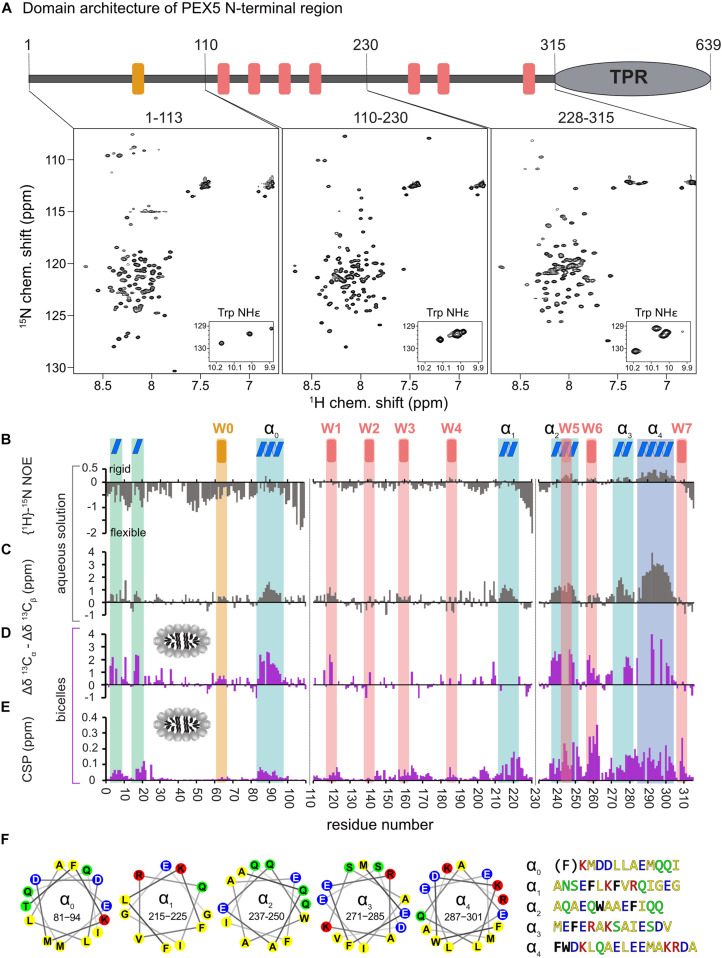
FIGURE 1.
Analysis of PEX5 NTD in the absence and presence of bicelles by NMR spectroscopy. (A) PEX5 NTD domain architecture and 1H–15N HSQC spectra of PEX5 constructs 1–113, 110–230, and 228–315 (B). {1H}-15N heteronuclear NOE experiments of the three constructs in aqueous solution. Negative values represent a highly flexible peptide backbone conformation. WF and the WF-like motif W0 are indicated by red or orange bars, respectively. (C) Less flexible regions were classified as helices α0–α4 (blue boxes) based on the secondary chemical shift (Δδ13Cα –Δδ13Cβ) (D). In the presence of bicelles the helices and two α-turns located in the first 20 aa, are stabilized which is shown by the secondary chemical shift (E). Chemical shift perturbations extracted from 1H-15N HSQC experiments demonstrate membrane binding which is mostly mediated by WF6 and the amphipathic helices α0–α4 visualized as helical wheels in (F). The residues are color coded in yellow for hydrophobic, green for polar, blue for negative charged and red for positive charged sidechains. Phe and Trp residues are shown in black and bold letters with exception of the Phe in α0 which does not contribute to the hydrophobic face of the helix.