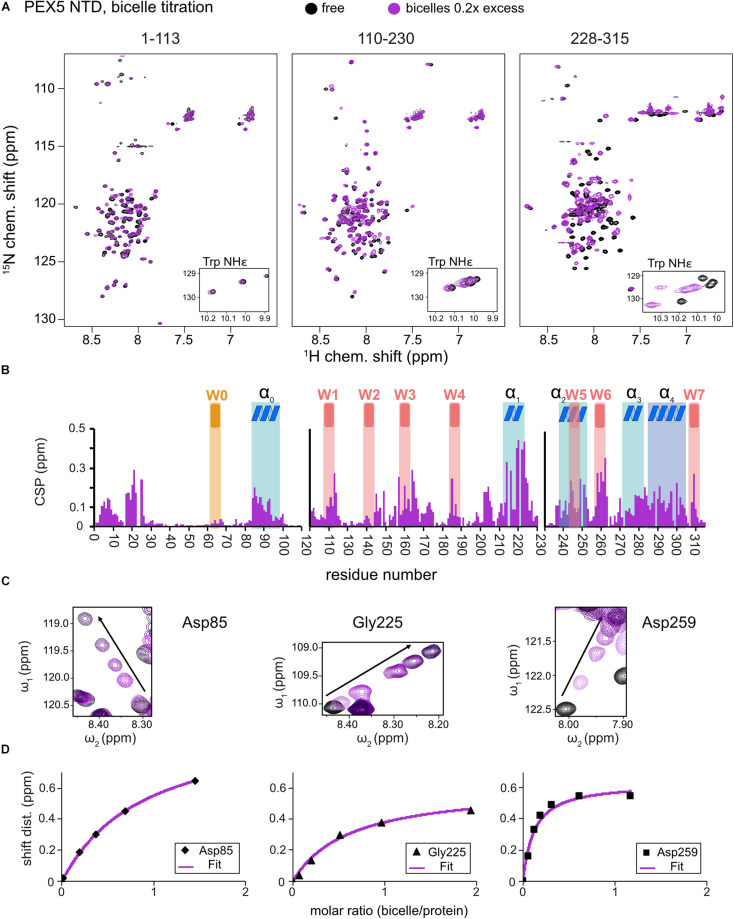
FIGURE 2.
PEX5 NTD – membrane binding. 1H,15N HSQC NMR spectra overlay (A) of free PEX5 1–113, 110–230, and 228–315 (back) and in the presence of 0.2x molar excess of DMPC/D7PC bicelles with a q-value of 0.2. The subconstruct 228–315 is largely affected by 0.2x molar excess while the constructs 1–113 and 110–230 show comparable effects at a molar excess of 0.7x and 0.9x (B). Tracing the chemical shift perturbations of largely affected residues Asp85, Gly225, and Asp259 of the subconstructs (C) and plotting shift distance against the molar ratio of bicelle to protein (D) Fitting of the NMR titration data (chemical shift difference to the free state) to a one-site binding model as a function of the molar bicelle:protein ratio (see section “Materials and Methods”).