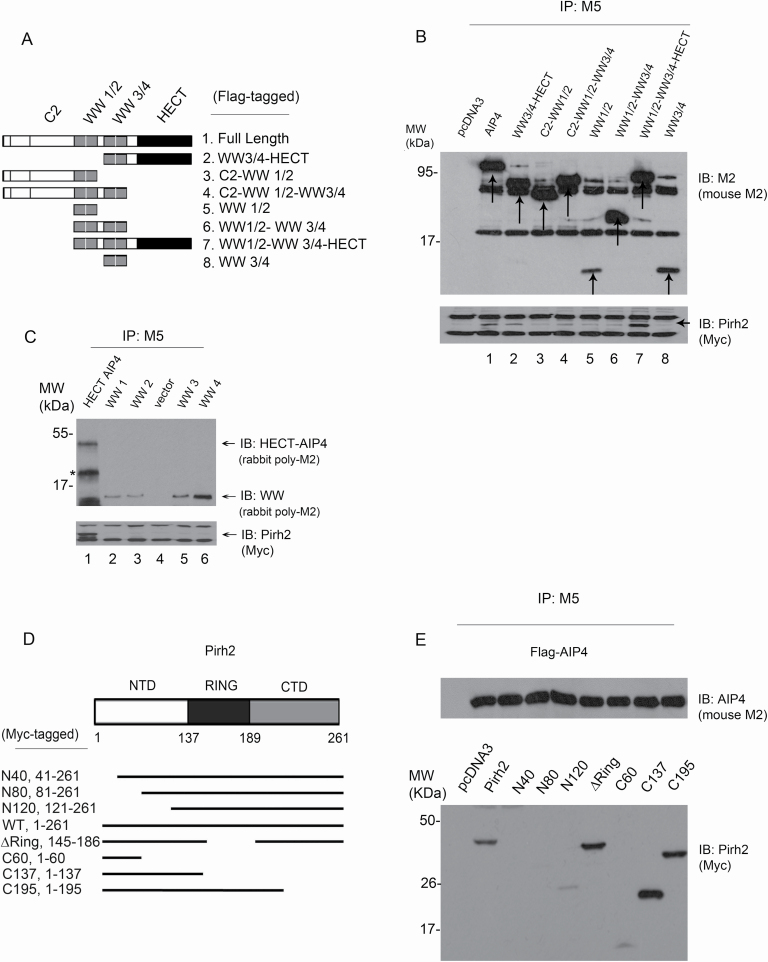
Figure 2.
Mapping the interaction sites of Pirh2 and AIP4. (A) Schematic representation of AIP4 different constructs designed for domain mapping. All constructs were expressed in a Flag-tagged vector. This figure was based on information obtained in Lallemand et al. (39). (B) Cell extracts prepared from H1299 cells ectopically expressing Myc-Pirh2 and Flag-AIP4 (full length and truncated constructs) were analyzed by co-IP. Cell lysates were immunoprecipitated using the M5 antibody (for AIP4) to pull down AIP4 constructs. Pirh2 binding was analyzed by immunoblotting using the Myc antibody for Myc-Pirh2 (bottom panel). A mouse monoclonal M2 antibody was used for immunoblotting to detect the expression of AIP4 constructs (top panel). (C) Plasmids expressing 3xFlag-tagged HECT-AIP4, WW1, WW2, WW3 and WW4 of AIP4 were transfected into H1299 cells together with Myc-Pirh2. Lysates were immunoprecipitated with an anti-Flag antibody (M5) to pull down AIP4 constructs, as indicated. Flag-tagged AIP4 constructs were visualized by immunoblotting with a rabbit polyclonal M2 antibody (top panel). The ability of Flag-AIP4 constructs to bind Myc-Pirh2 was analyzed by immunoblotting with an antibody against Myc (Myc-Pirh2, bottom panel). (D) Schematic representation of Pirh2 different constructs designed for mapping. All constructs were expressed in the Myc-tagged vector. (E) Cell extracts prepared from H1299 cells ectopically expressing Flag-AIP4 and Myc-Pirh2 (full length and truncated constructs) were analyzed by co-IP. Cell lysates were immunoprecipitated using the M5 antibody to pull down AIP4. Pirh2 binding was analyzed by immunoblotting using the Myc antibody for Myc-Pirh2 constructs (bottom panel). A mouse monoclonal M2 antibody was also used for immunoblotting to detect AIP4 expression (top panel).