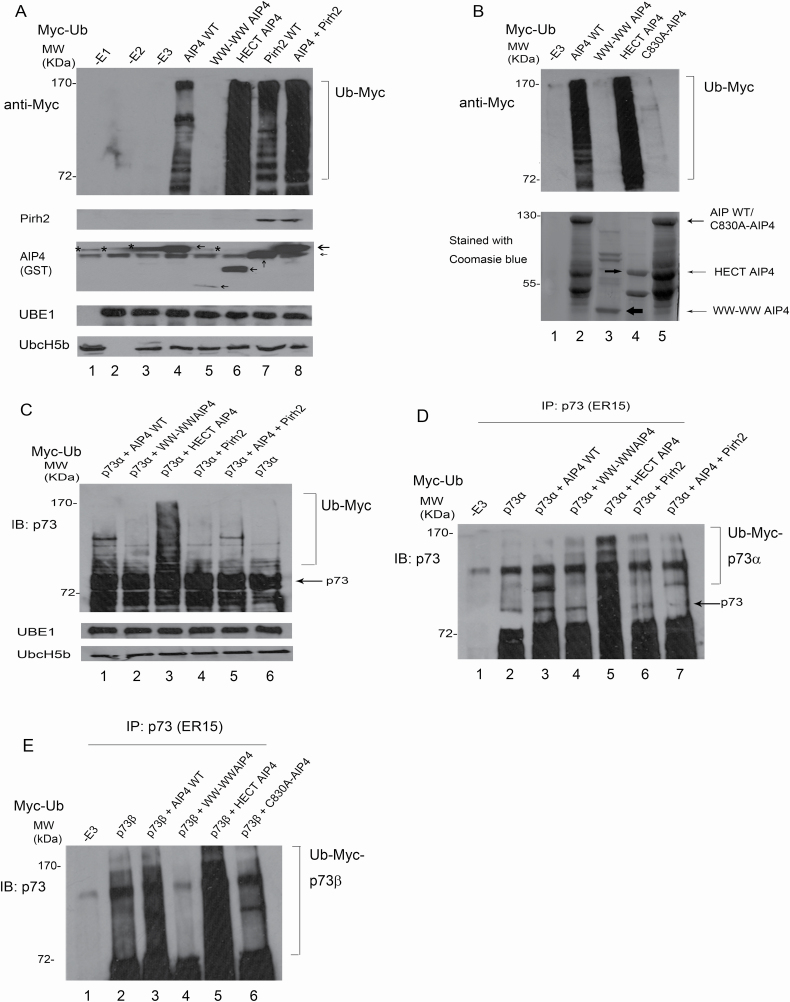
Figure 6.
Pirh2 promotes AIP4 self-ubiquitination and regulates AIP4-mediated p73 ubiquitination in vitro. (A) GST-AIP4 or various GST-AIP4 deletion constructs were evaluated for their E3 ligase ability in the presence of E1, E2 and Myc-Ub, as indicated. The levels of protein ubiquitination were analyzed by western blotting, as shown. The levels of Pirh2, AIP4, UBE1 and Ubc-H5b were detected by western blotting. * shows the background signals of anti-GST antibodies. (B) Similar to (A), except that GST-C830A-AIP4 was used (top panel). GST-AIP4 or GST-AIP4 mutant constructs were purified from E. coli. Prior to ubiquitination assays, constructs were run on SDS–PAGE gel and stained with Coomassie blue (bottom panel). (C) GST HECT-AIP4 was evaluated for its ability to ubiquitinate p73α in vitro. His-p73α, GST-AIP4 or various GST-AIP4 constructs, or GST-Pirh2, E1 and E2 were added to the in vitro ubiquitination reaction and analyzed by western blotting with a p73-specific antibody to detect p73 ubiquitination in vitro. Direct western blots for UBE1 and Ubc-H5b are shown in the lower panel. (D) Similar to (C), except that the reaction after ubiquitination was immunoprecipitated with a p73-specific antibody and analyzed by immunoblotting with a p73-specific antibody as indicated. (E) Similar to (D), except that His-p73β was used.