Figure 4: An actin-remodeling network providing mechanical plasticity ensures embryo elongation.
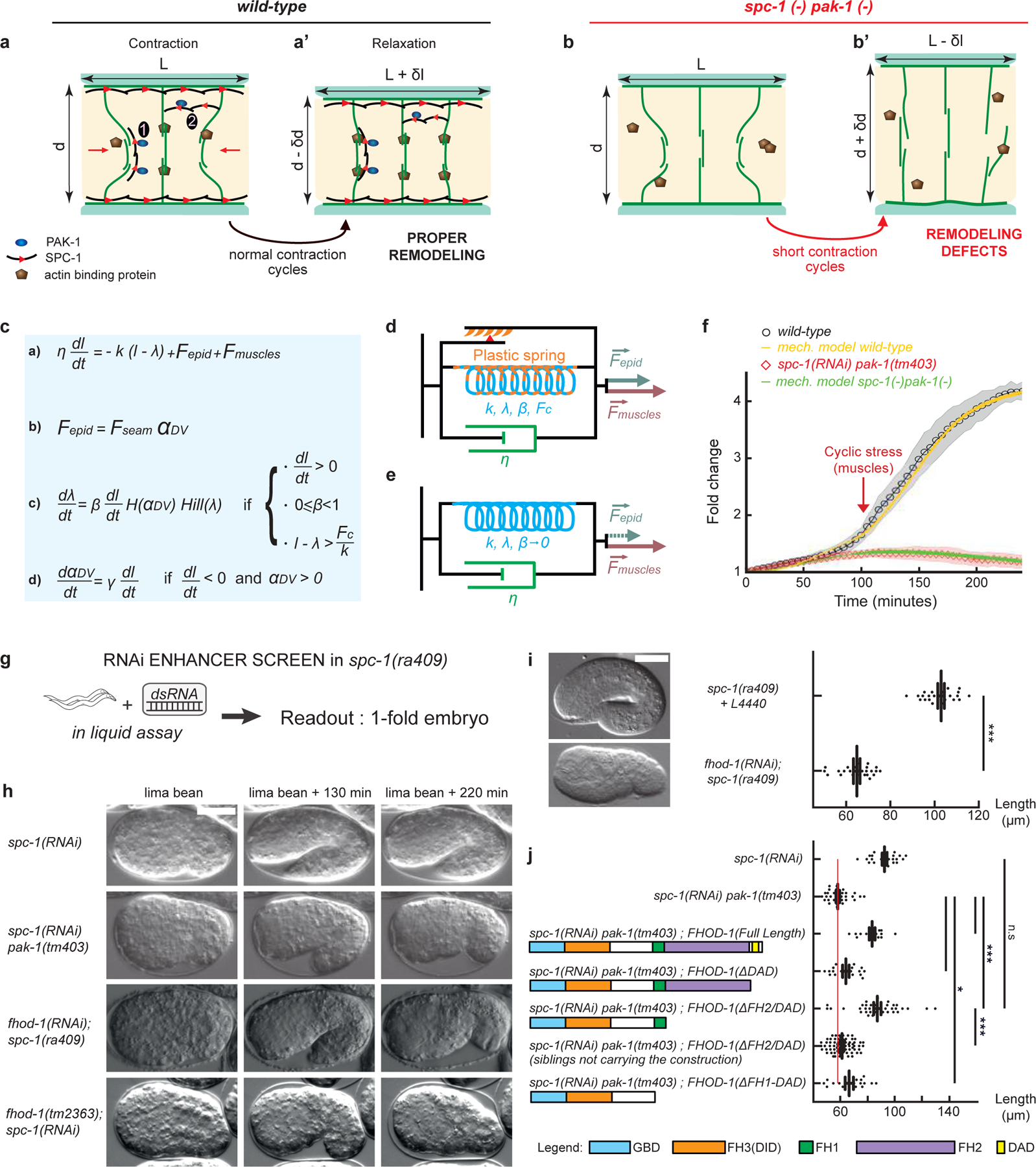
(a-b’) Cellular model of embryo elongation. (a-a’) In control embryos, muscle contractions (red arrows) provoke actin filament shortening in the dorso-ventral epidermis, probably through sliding or shortening, followed by SPC-1/PAK-1-dependent actin stabilization. Whether spectrin is found along (scenario 1) or between (scenario 2) actin filaments is unknown (a). (b-b’) In spc-1 pak-1 deficient embryos, actin remodeling goes uncontrolled. (c-f) Viscoplastic mechanical model of embryo elongation. The embryo is represented as a Kelvin-Voigt solid (spring stiffness k, resting length λ, viscosity η) submitted to the forces Fepid and Fmuscle. System equations for the model. (d) Wild-type case: an increasing resting length during stretching phases imparts mechanical plasticity. (e) spc-1 pak-1 mutants: Fepid progressively decreases. (f) Comparison of experimental and predicted elongation curves taking the constitutive equations shown in (c). (g) A retraction screen in a spc-1 mutant identifies fhod-1. (h) Snapshot at three time-points of spc-1 deficient embryos in control, pak-1 or fhod-1 backgrounds; (i) terminal body length at hatching: spc-1(ra409) after feeding on L4440 control (n=21), or fhod-1(RNAi) (n=25) bacteria. (j) Pdpy-7 driven epidermis expression of truncated FHOD-1 variants and terminal body length at hatching: spc-1(RNAi)(n=26); spc-1(RNAi)pak-1(tm403) no transgene (n=36), FHOD-1(full length) (n=16), FHOD-1(ΔDAD) (n=17), FHOD-1(ΔFH2-DAD) (n=38) and non-transgenic siblings (n=78), FHOD-1(ΔFH1-FH2-DAD) (n=18). Scale bar, 15 μm. Error bars, SEM. P values: *<0,05; **<0,001; ***<0,0001; ns, not significant.
