Figure 1.
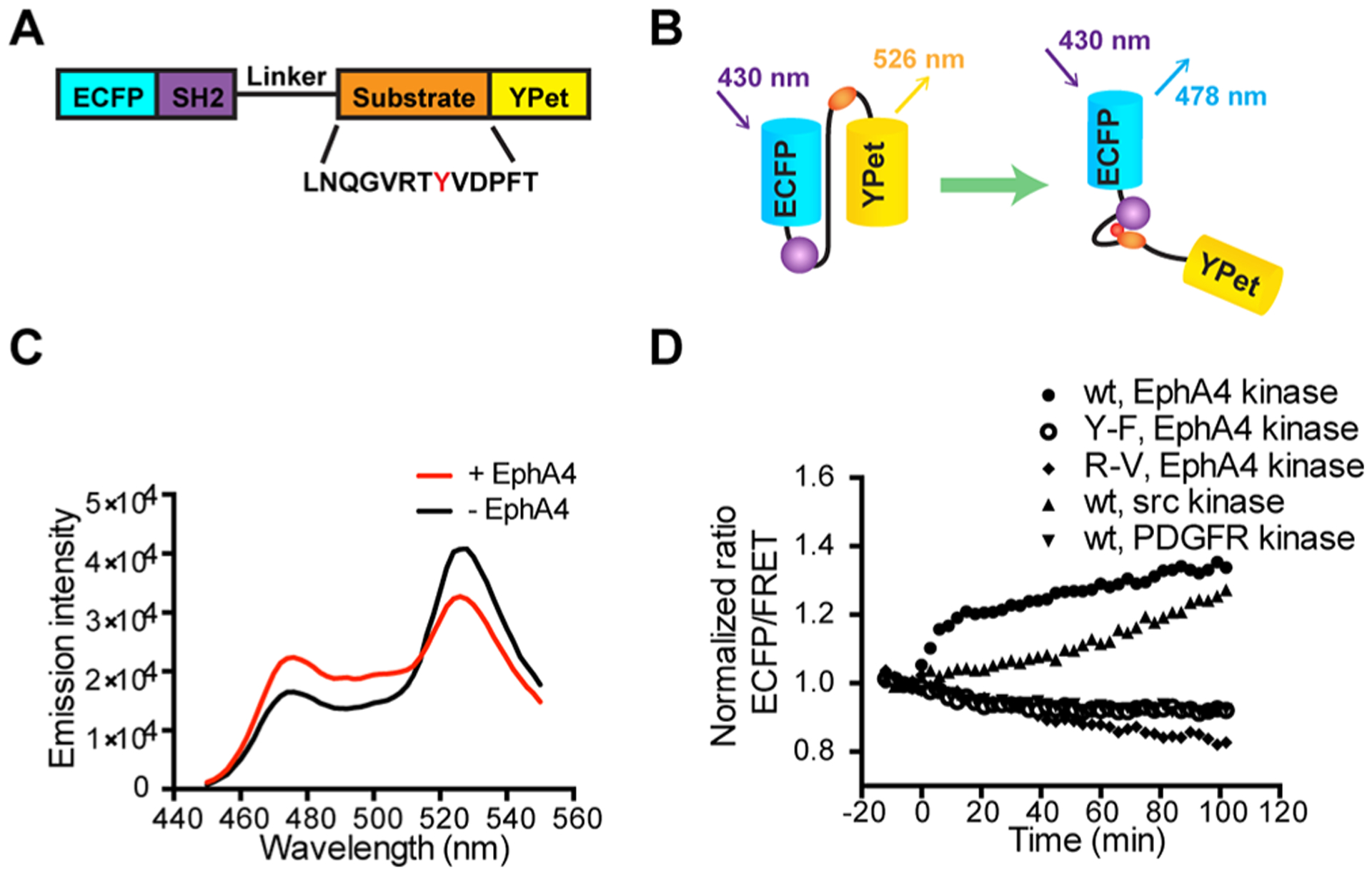
Design and in vitro characterization of FRET-based EphA4 biosensor. (A) The EphA4 biosensor is composed of ECFP, the c-Src SH2 domain, a flexible linker (see Materials and Methods), the EphA4 substrate peptide containing Tyr596 (indicated as a red Y) and YPet. (B) Design principle of the EphA4 biosensor. Active EphA4 phosphorylates the substrate peptide of the EphA4 biosensor, which then intramolecularly binds to the SH2 domain, causing a decrease in the FRET signal. (C) Emission spectra of the purified EphA4 biosensor before (black) and after (red) phosphorylation by EphA4 for 2 h. (D) Time courses of the ECFP/FRET emission ratio of the EphA4 biosensor (1 μM) before and after incubation with EphA4 (1 μg/mL), Src kinase (1 μg/mL), or PDGFR (1 μg/mL) (N = 3). Time courses of EphA4 Y596F (Y–F) mutant biosensor (1 μM) and SH2 R175 V (R–V) mutant biosensor (1 μM) before and after EphA4 stimulation is also shown (N = 3). The ECFP/FRET ratios were normalized to the average values before enzyme incubation. “N” means the number of independent experiments.
