Figure 2.
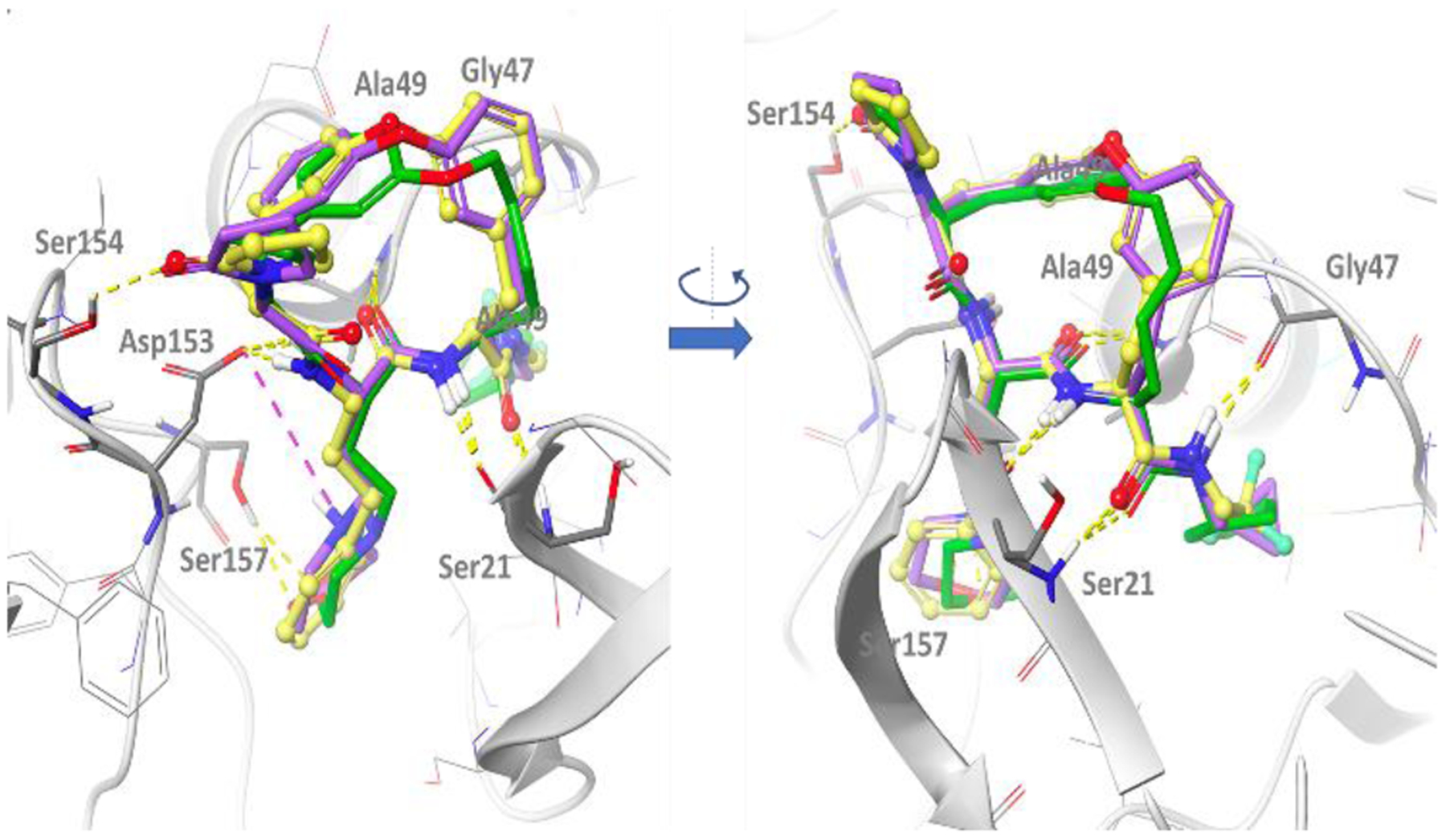
Docking model of macrocyclic peptides in Pf20S. The structure of Pf20S β5–6 was obtained by homology modeling based on yeast 20S β5–6 (3MG4), and then refined using molecular dynamics simulations. The amide bonds of all compounds form 5 well conserved hydrogen bonds with the backbone of Ser21, Gly47 and Ala49 as well as the side chain of Asp153. Additionally, the pyrrolidone in P5 of compounds 2, 3 and TDI-8304 forms a hydrogen bond with Ser154, and the morpholino oxygen in compound 3 and TDI-8304 form a hydrogen bond with hydroxyl group of Ser157 in β6. For compound 3, the protonated form of the morpholine has been represented to illustrate the possible salt bridge with Asp153, but the neutral form might predominate at relevant pH (predicted pKa ~ 6.6). Compound 2 is shown in yellow, compound 3 in purple and TDI-8304 in green.
