Figure 5.
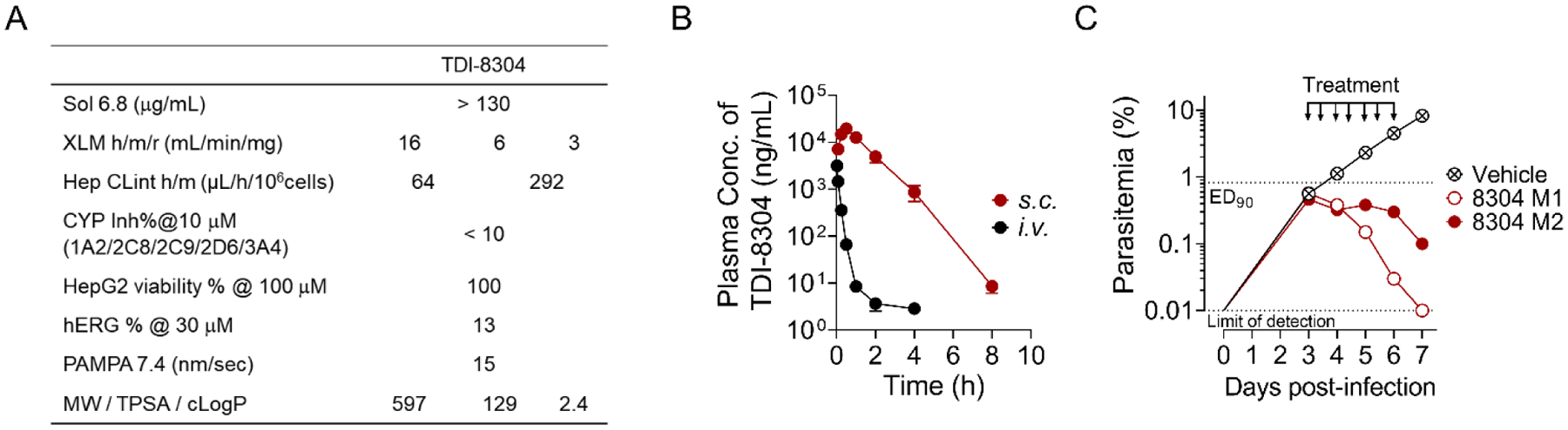
Pharmacokinetic properties of TDI-8304 and its efficacy in humanized, P. falciparum-infected mice. A) PK properties of TDI-8304. B) In vivo PK of TDI-8304 administrated i.v. (1 mg/kg) or s.c. (100 mg/kg). C) Parasitemia reduction of P. falciparum Pf3D70087/N9 in NOD-SCID IL-2R-null mice transfused with human erythrocytes. TDI-8304 was given twice-daily by s.c. (100 mg/kg) on days 3 through 6. Two mice were used in each group, each serially sampled on days 3 to 7. Percentage parasitemia was calculated by acquiring a minimum number of 500 parasitized erythrocytes.
