Fig. 2.
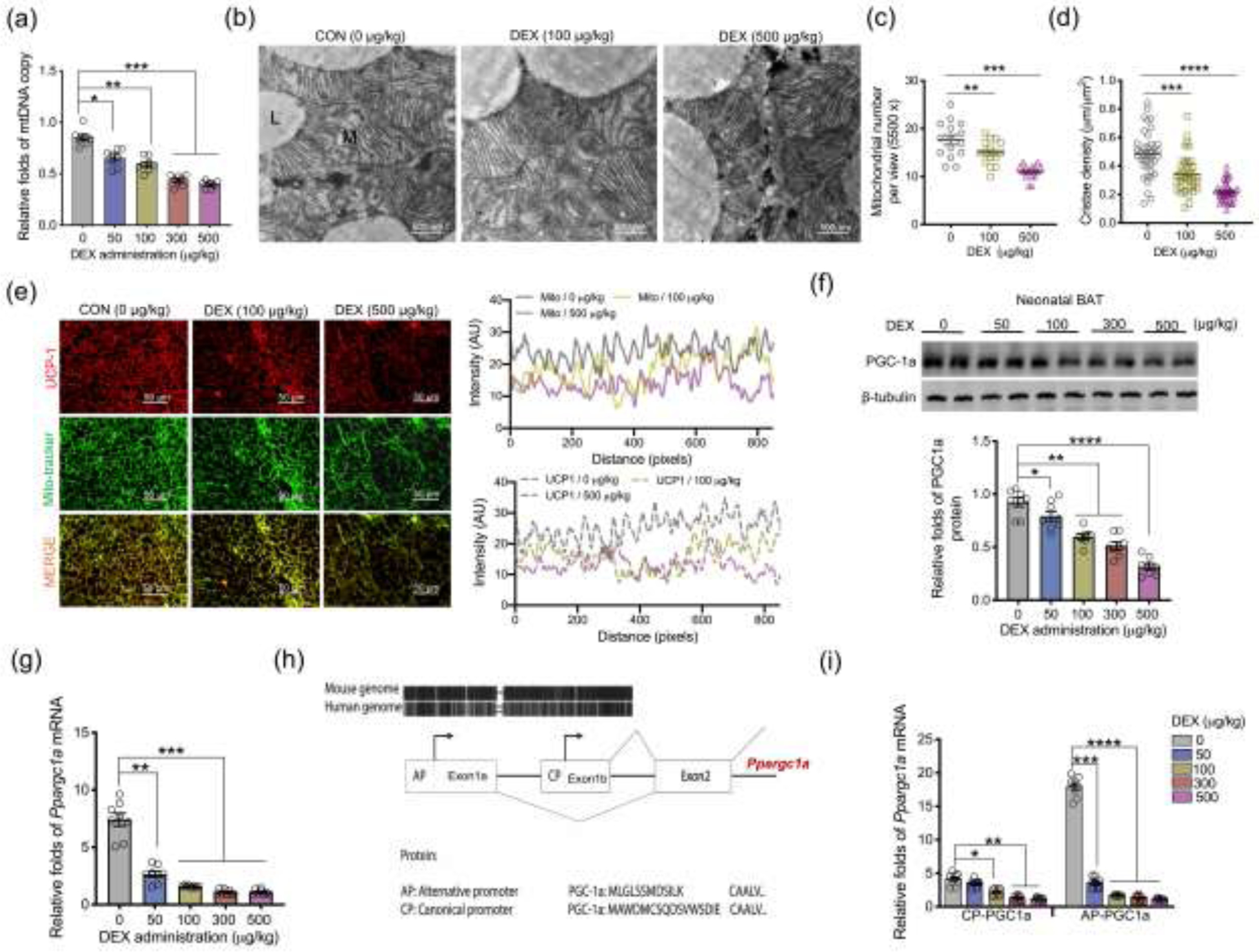
Excessive glucocorticoid exposures during pregnancy inhibit mitochondrial biogenesis and Ppargc1a transcription from the alternative promoter in BAT of neonatal offspring. (a–j) BAT was collected and analyzed in neonates on postnatal day 1 (P1) born from maternal mice exposed synthetic glucocorticoids, dexamethasone (DEX), in the last trimester of pregnancy. (a) Mitochondrial DNA (mtDNA) copy number in neonatal BAT (n = 8 in each group). (b–d) Transmission electron microscopy analyzing mitochondrial number and cristae density in neonatal BAT (n = 4 in each group). “L” represents lipid droplet, and “M” represents mitochondria. Scale bar = 500 nm. (e) Immunostaining measurements of UCP-1 (red) and mitochondria (mito-tracker, green) in neonatal BAT. Intensity of UCP-1 and mito-tracker were quantified by Image-J (n = 4 in each group). Scale bar: 50 μm. (f) Immunoblotting measurement of PGC-1a protein content in neonatal BAT. β-tubulin was used as a loading control (n = 8 in each group). (g) mRNA expression of Ppargc1a in neonatal BAT. Expression was normalized to 18S rRNA (n = 8 in each group). (h) Diagram shows the canonical promoter (CP) and alternative promoter (AP) of Ppargc1a are highly conserved between human and mice. Except for transcriptional difference in the first exon, transcription and encoded amino acids from other exons are the same between Ppargc1a CP and AP. (i) Ppargc1a mRNA expression transcribed from CP and AP in BAT of neonates born to DEX mothers. mRNA expression was normalized to 18S rRNA (n = 8 in each group). Data are mean ± SEM and each dot represents one replicate (each pregnancy); *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; unpaired one-way ANOVA multiple test was used in analyses.
