Figure 6. Ablation of the HS-Tie1 interaction in vivo causes aberrant retinal vascularization and loss of endothelial pro-survival signaling.
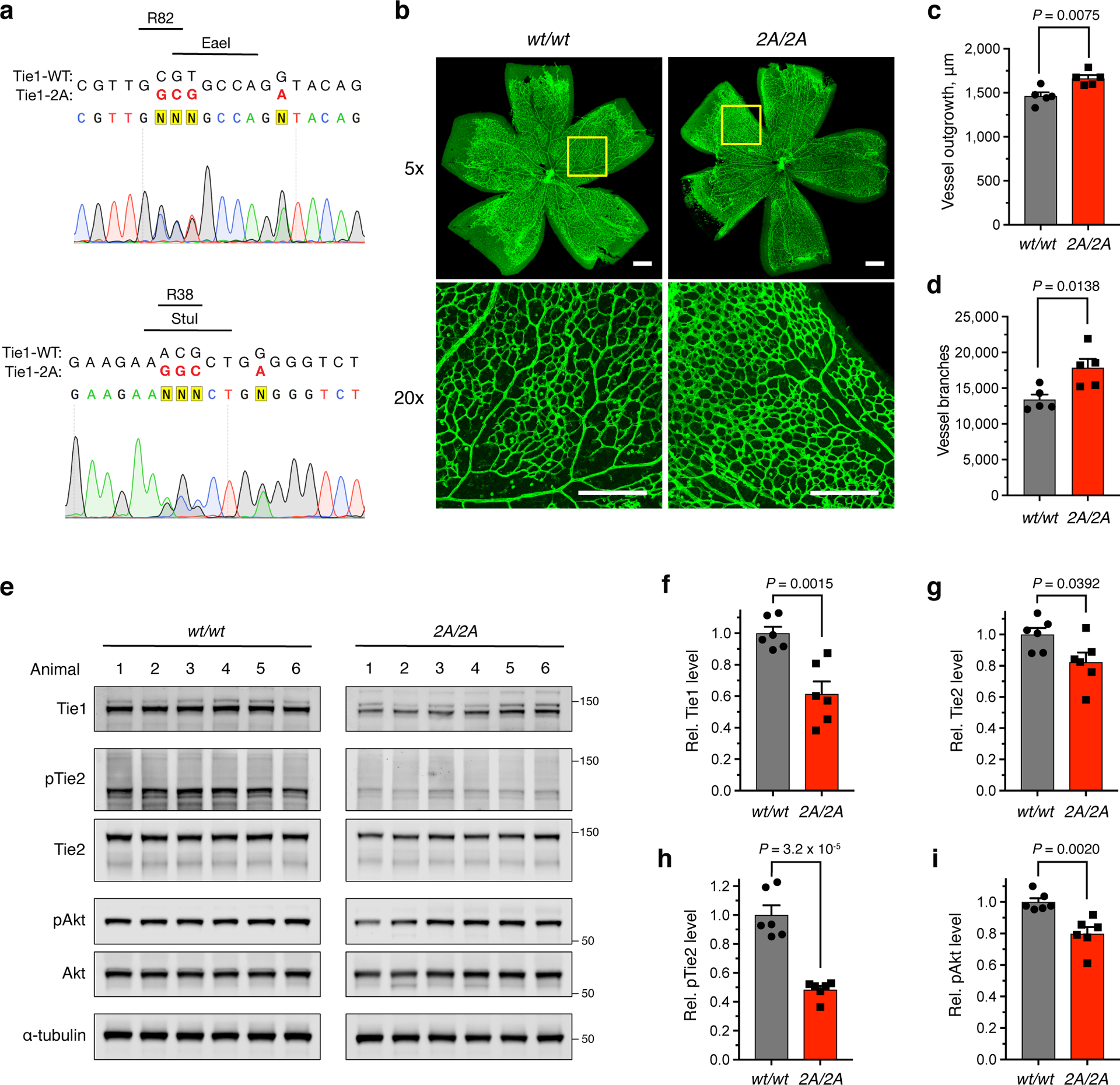
(a) Sequencing results from a heterozygous Tie12A/wt mouse. Mutated nucleobases are shown in red with the mutated amino acid residues, newly generated restriction enzyme sites, and removed NGG protospacer adjacent motifs displayed above the sequence. (b) Representative images and (c,d) quantification of retinal blood vessel radial outgrowth and vessel branching from 7-day-old Tie1-2A and wild-type littermates using the endothelial-specific isolectin GS-IB4 (green), scale bar = 200 μm, n = 5 retina per genotype. (e) Western blotting and (f-i) quantification of total protein and phosphoprotein levels within the Ang/Tie pathway using lung tissue samples from Tie12A/2A and wild-type 4-month-old littermates, n = 6 animals per genotype. All protein levels are normalized to the corresponding ɑ-tubulin loading control, and all phosphoprotein levels are normalized to the corresponding total protein level. Proteins were imaged on the same blot to allow for direct comparison between samples. Data are reported relative to the wild-type control. All data represent mean ± s.e.m., unpaired, two-tailed Student’s t test.
