Keywords: cirrhosis, endoscopic ultrasound, metabolomics, portal hypertension, portal vein
Abstract
Portal and hepatic circulation can now be safely accessed using endoscopic ultrasound (EUS). EUS-guided needle access of the portal vein is performed clinically at select tertiary centers for measurement of portal pressure gradients in patients with chronic liver disease and sampling of portal venous thrombus to diagnose malignancy. We propose that this novel clinical technique can be applied in research studies to allow blood collection from and profiling of portal and hepatic circulation. In this technical report, we present and highlight the technical aspects, feasibility, and safety of EUS: guided portal venous blood collection. As a proof of the concept and the utility of this technique in metabolic research and biomarker assessment and discovery, we present a pilot metabolite profiling study of portal venous blood in a small cohort of patients with cirrhosis and a comparison with a group without cirrhosis. Despite the very small diameter of the endoscopic needle used for the blood collection, the portal samples have the same quality as those collected from systemic circulation, and they can be used for the same downstream applications. Finally, we propose an analytical workflow to screen for promising metabolites that could qualify for further studies to determine their utility as sensitive, early candidate biomarkers of hepatic fibrosis, portal shunt, and hypertension. We hope that this report could stimulate and facilitate the widespread use of EUS-guided techniques for the profiling of portal circulation, which could potentially open a new field of scientific inquiry.
NEW & NOTEWORTHY The technical aspects, feasibility, and safety of endoscopic ultrasound (EUS)-guided needle access for portal venous blood collection are presented in this technical report. Despite the very small diameter of the endoscopic needle, portal blood samples have the same quality as those collected from systemic circulation. As a proof of the concept and the utility of this technique in metabolic research and biomarker assessment and discovery, we present a pilot metabolite profiling study of portal venous blood in a small cohort of patients with cirrhosis and a comparison with a group without cirrhosis.
INTRODUCTION
Portal circulation content has profound effects on liver metabolism and reflects directly the output from the spleen, pancreas, and gastrointestinal GI system, including the microbiome. Absorbed nutrients, xenobiotic substances, and metabolic products of normal cells of the GI organs or from microbiota and cancerous cells reach the liver through the portal system, which supplies ∼75% of the total liver blood flow (9). There, they undergo metabolic biotransformation, which affects the liver itself but also determines the hepatic output, thereby affecting the other organs of the body. Furthermore, nutrient sensing by the portal vein itself has been considered as another mode of communication between the gut and distant organs, including the brain (10). Historically, the limited accessibility to portal and hepatic vessels and the lack of widespread application of minimally invasive sampling techniques are the main challenges that prevent the direct characterization of hepatic input and output in human patients. As a result, the compositional signatures of the portal vein, the primary hepatic input, remain an opaque box for which very little is known in health and disease states. In addition, the hepatic output, which directly reflects liver function and its metabolic state, is usually inferred by systemic circulation signatures, generated from samples collected from vessels distant from the liver, whose composition does not reflect the hepatic output alone.
Portal and hepatic circulation can now be safely accessed using endoscopic ultrasound (EUS). EUS-guided needle access of the portal vein is already performed clinically at select tertiary centers for measurement of portal pressure gradients in patients with chronic liver disease and sampling of portal venous thrombus to diagnose malignancy (6, 12). It has also been used in patients with pancreatic cancer for the sampling and characterization of circulating tumor cells (5). We have recently embarked on a novel research program, with the overarching goal to utilize this technique for the profiling of portal circulation in human patients as a novel way to enhance our understanding of the pathogenesis of gastrointestinal cancers and metabolic diseases and enable the development of new diagnostic/screening tools and therapeutics.
The aim of this technical report is to demonstrate, discuss, and highlight the technical aspects and the feasibility and safety of endoscopic ultrasound (EUS)-guided blood collection from the portal circulation. As a proof of the concept for the utility of this technique in metabolic research and biomarker discovery and assessment, we present a pilot metabolomic profiling study of portal venous blood in a small cohort of patients with cirrhosis that was compared with a group of patients with pancreatic ductal adenocarcinoma.
METHODS
This study was approved by the Institutional Review Board (IRB) of Brigham & Women’s Hospital and was conducted between October 2018 and October 2019. Patients were consented for EUS-guided portal venous collection and concomitant peripheral venous blood collection. Inclusion criteria were adult age (older than 18 yr) and referral for a clinically indicated endoscopic ultrasound procedure. Exclusion criteria included coagulopathy or bleeding tendency [international normalized ratio (INR) > 1.6 and platelet count < 50,000/μL], blood transfusion within 30 days, and inability to provide consent. All enrolled patients were either observed as inpatients (if they were already hospitalized) or seen as outpatients within 2 wk of their procedure. Adverse events such as internal bleeding (e.g., liver hematoma), infection, perforation, thrombus formation, and severe pain were recorded.
EUS TECHNIQUE FOR SAMPLING THE PORTAL CIRCULATION
All procedures were performed under general anesthesia by a single operator (MR). Prior to the procedure, a standard 22-gauge FNA needle (Expect Slimline EUS Aspiration Needle, Boston Scientific, Marlborough, MA) was prepared by flushing with heparin lock flush solution (10 U/mL) and reinserting the stylet (Fig. 1) . A linear echoendoscope (Olympus GF-UCT180; Olympus, Center Valley, PA) was advanced through the mouth and into the stomach to assess the portal venous circulation, including the portal veins and portal collateral vessels if present. The flow signal of these vessels was confirmed by Doppler interrogation. If patients were clinically referred for EUS-guided coil obliteration of their portal collateral vessels (i.e., gastric varices, which are prone to bleeding), the collateral vessels were preferentially targeted for needle puncture (standard 22-gauge FNA needle; Expect Slimline EUS Aspiration Needle, Boston Scientific) (4). For all other study patients, the same 22-gauge FNA needle was advanced under EUS guidance across the gastric wall, across the liver parenchyma, into the left portal vein. Upon needle entry into the targeted vessel, the stylet was removed and a BD Vacutainer holder with multiple sample adapter (Becton-Dickinson, Franklin Lakes, NJ) attached to the EUS needle handle. An evacuated blood collection tube was applied for plasma collection. A second tube was then attached for serum collection. Importantly, blood was not collected via manual aspiration into a syringe. In cases of direct portal venipuncture, the needle was slowly withdrawn across the hepatic parenchyma over 1–2 min under EUS Doppler examination to ensure tamponade by hepatic parenchyma. In cases of variceal puncture, coiling was subsequently performed per original clinical request with a previously published technique (4).
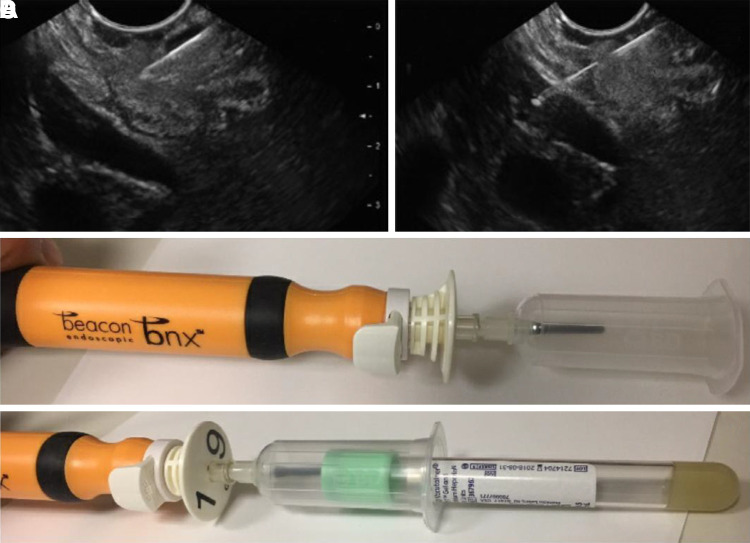
Fig. 1.
The 2 ends of the standard 25- or 22-gauge endoscopic ultrasound (EUS) needle that are used for the blood collection. Once the needle tip is confirmed within the portal vein by ultrasound (A and B), the stylet is withdrawn from the needle lumen, and a vacutainer is attached to the back end of the needle (C and D). Standard collection tubes are then inserted into the vacutainer to collect a total of 20 mL maximum.
SPECIMEN PROCESSING
Blood samples were immediately placed in ice and processed within 2 h using standard protocols. Briefly, the plasma samples were centrifuged at 1,300 RCF at room temperature. For serum, the samples were incubated at room temperature for 45 min and subsequently centrifuged at 1,300 RCF for 15 min. The supernatant was then transferred to a cryovial and stored at −80°C. Liquid chromatography-tandem mass spectrometry was used to catalog the detectable metabolites in the portal and systemic circulation.
PROOF OF CONCEPT STUDY RESULTS
EUS-guided portal venous sampling was performed on a total of 12 consecutive patients (Table 1). Five patients had cirrhosis (mean age 56 ± 11.2 yr; 3 with alcoholic liver disease, 2 with NAFLD), and seven had pancreatic cancer (mean age 61 ± 9.7 yr). Portal venous blood was successfully obtained in 100% (12 of 12) of the patients. All patients did well during and after the procedure, without bleeding, infection, perforation, thrombus formation, severe pain, or other postprocedural complications at a mean follow-up of 4.2 wk (±0.8 wk; Table 2).
Table 1.
Baseline patient demographics
| Cirrhosis Patients (n = 5 patients) | Pancreatic Cancer Patients (n = 7 patients) | P Value | |
|---|---|---|---|
| Age, yr | 56.0 (± 11.2) | 61 (± 9.7) | 0.76 |
| Males, % | 60 (n = 3) | 57 (n = 4 patients) | 0.56 |
| Disease etiology, % | Alcohol: 60 (n = 3 patients) NAFLD: 40 (n = 2 patients) |
PDAC: 100% (n = 7 patients) | |
| Disease stage, % | Compensated: 60 (n = 3 patients) Decompensated: 40 (n = 2 patients) |
Stage 2: 29 (n = 2 patients) Stage 3: 29 (n = 2 patients) Stage 4: 42 (n = 3 patients) |
|
| Current smoker, % | 40 (n = 2 patients) | 14 (n = 1 patients) | 0.45 |
| BMI, kg/m2 | 24.3 (± 5.4) | 28.7 (± 6.8) | 0.67 |
| History of diabetes, % | 40 (n = 2 patients) | 57% (n = 4 patients) | 0.87 |
Total patients, n = 12. NAFLD, nonalcoholic fatty liver disease; PDAC, pancreatic ductal adenocarcinoma.
Table 2.
Technical success and adverse events
| Parameter | Value |
|---|---|
| Overall technical success (completion of portal vein aspiration) | 100% (12 of 12) |
| Absence of hemolysis | 100% (12 of 12) |
| Adverse events (%, immediate and delayed) | Total 0% |
| Bleeding | 0% |
| Perforation | 0% |
| Thrombus formation | 0% |
| Severe abdominal pain | 0% |
| Infection | 0% |
| Clinical follow-up (wk) | 4.2 (±0.8) |
Portal venous sampling volume was >2.5 mL in all cases. All samples passed quality control, especially for the presence of hemolysis, that would render the performance of downstream profiling studies challenging. We detected 397 unique metabolites of known identity, which is comparable with what we detected in peripheral blood analyses. We detected more than 35,000 metabolites total, and the distribution of their portal levels compared with systemic circulation is shown in Fig. 2. As a proof of concept study, we then further analyzed the metabolomic data to determine whether this approach could be used to screen for metabolites that could qualify for further studies that would determine their utility as early biomarkers of cirrhosis. We prospectively defined three consecutive rounds of analysis with the following specific criteria.
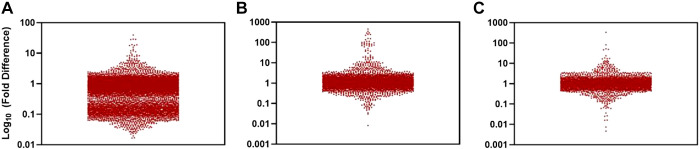
Fig. 2.
Proof-of-concept metabolite profiling of samples collected from portal circulation in patients with cirrhosis. Each point represents 1 metabolite and the 10th logarithm of the times that its levels in the portal are different compared with peripheral blood. Comparison of amino acids, amino acid metabolites, acylcarnitines, dipeptides, and other cationic polar metabolites (A), free fatty acids, lipid-derived mediators, bile acids, and metabolites of intermediate polarity (B), and sugars, organic acids, purines, pyrimidines, and other anionic polar metabolites (C).
Round 1
First, we kept in the analysis only those molecules that in the control group had statistically different levels between the portal and systemic circulation (39 statistically significant metabolites, P < 0.05, and an additional 25 marginally significant with P values between 0.05 and 0.1). The rationale behind this criterion is that it likely captures the metabolites for which the normal liver has a significant impact on their levels. We were especially interested in those metabolites whose levels were higher in the portal compared with systemic circulation, as this supports the assumption of hepatic filtration and the impact of the liver on portal signatures (11 metabolites, P < 0.05; 7 more with 0.05 < P < 0.1).
Round 2
Next, we took these metabolites and examined the portal versus systemic levels in the subjects with cirrhosis. The goal was to eliminate the ones that exhibited different levels between portal and systemic circulation. Thus, we chose only those metabolites that exhibited no difference between portal and systemic circulation in patients with cirrhosis. The assumption in this criterion is that the similar portal and systemic levels (as opposed to the different levels in the control group) are more likely to result from the spillover of the portal contents into the system circulation. No metabolite from round 2 was eliminated (thus 11 metabolites with P < 0.05; 7 more with 0.05 < P < 0.1 qualified for the next step).
Round 3
The final selection was based on the levels in systemic circulation between the control group and the subjects with cirrhosis. We chose those metabolites that were different between the two groups, as this is a requisite criterion for a biomarker (i.e., to differentiate in the peripheral circulation the cirrhosis group from the controls). Five metabolites met all these criteria and had statistically significantly higher levels in patients with cirrhosis compared with controls: N-lauroylglycine (HMDB13272), 1-methylguanosine (HMDB01563), 12,13-DiHOME (HMDB04705), 9,10-DiHOME (HMDB04704) and TUDCA (tauroursodeoxycholic acid; HMDB00874). In addition, methyl N-methylanthranilate (HMDB34169), guanidinoacetic acid (HMDB00128), and glycochenodeoxycholic acid glycine conjugate (HMDB00637) were marginally significant (0.05 < P < 0.1).
Thus, with this approach we would select 1.2% (5 of 397) for further validation studies that would examine their utility as disease biomarkers (and an additional 0.7% if we included the 3 additional metabolites with 0.05 < P < 0.1). In contrast, if we selected candidates based on the commonly used strategy of comparing their levels only in systemic circulation, this study would conclude that 15.6% of metabolites could potentially be disease biomarkers (since there were 62 out of 397 metabolites with P < 0.05). There was also an additional 6.5% of metabolites (26 of 397) with 0.05 < P < 0.1.
DISCUSSION
Our data show that EUS-guided sampling of portal venous blood in humans appears to be feasible and safe. Importantly, the EUS technique that we described uses a smaller needle than what was previously reported for collection of circulating tumor cells (5) and may theoretically be safer. Importantly, despite the smaller needle lumen, the quality of the samples is similar to that of samples collected from the systemic circulation and can be used for the same downstream applications that are used to analyze systemic blood samples. Although in this report we performed a proof-of-concept metabolomic analysis, the sample volume appears to be sufficient for any other downstream application.
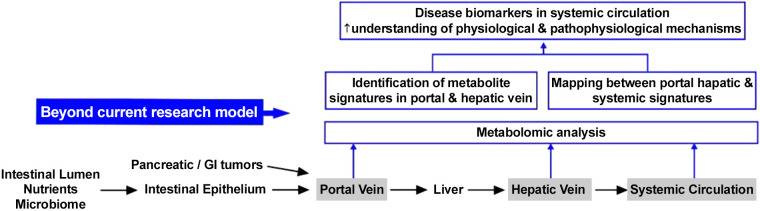
Highly parallel assays such as the metabolomic profiling of the portal venous circulation could potentially elucidate the gut-liver axis, the communication of the liver with other organs, and the potential impact of gut microbiome in various disease states (13). This in vivo experimental setup in humans may allow us to gain novel insights into liver function and metabolism and fundamental physiological processes such as the hepatic filtration of the contents of the gut-liver axis or the determination of portal vein signatures that are altered, obscured, or erased by or persist despite the first-pass liver metabolism. Our approach may complement and enhance the analysis of peripheral blood samples, which is known as “liquid biopsy” that has recently emerged as a promising tool for diagnosis, risk stratification, disease monitoring, and ultimately, personalized treatment recommendations (2, 3, 8). Mapping portal and systemic signatures (Fig. 3) may offer one of the most promising ways to identify relevant peripheral signatures and biomarkers that could eventually be exploited for earlier and noninvasive diagnosis of disease progression. It may also offer a more reliable way to associate patient-reported outcomes and symptoms with specific metabolites or metabolic phenotypes.
Fig. 3.
Endoscopic ultrasound (EUS)-guided sampling and profiling of portal circulation can expand the current research model, in which liver function and portal signatures are inferred by the profile of samples collected from systemic veins.
As a proof of this concept, in our pilot cohort of patients with cirrhosis, we attempted to test whether this approach could identify metabolites that could be studied further as potential candidate biomarkers of cirrhosis and hepatic dysfunction. In this small pilot study, five metabolites were statistically different between the cirrhotic patients and controls, whereas three additional metabolites were marginally significant. These candidates included N-lauroglycine and TUDCA, two metabolites that have also been reported in metabolite profiling studies of the systemic circulation in patients with cirrhosis (7, 11, 14). Because the proposed approach is based on a series of prospectively defined rational criteria about the pre- and posthepatic concentration levels of each metabolite, the proposed approach may provide a way to refine and focus on specific candidate biomarkers and to offer more confidence in their clinical utility.
The goal of this report was to highlight the feasibility and safety of EUS-guided portal sampling for clinical research studies. The metabolite profiling was a proof of concept exercise that paves the way for studies with larger sample size that will allow more sophisticated statistical modeling and adjustment for multiple comparisons. The data from this report can provide valuable information about the design and the required statistical power of these studies. One limitation of the proposed approach is that it is unlikely for any comparison to involve a group of healthy controls. Thus, the selection of the control group is an important consideration in the study design and might frequently pose interpretational challenges. Another limitation is the procedural scalability, given the need for expertise in interventional advanced endoscopic techniques. We anticipate that this latter problem will be improved because this expertise is available at select academic medical centers, and recent developments include the FDA authorization of devices for portal circulation access (1).
In conclusion, the key message of this study is the demonstration and application of a novel clinical technique with the potential to open up a new field of scientific inquiry that is based on the profiling of portal circulation. We hope that this pilot feasibility study could stimulate and facilitate the widespread use of EUS-guided techniques for the profiling of portal circulation, which could enhance and facilitate studies on gut-liver axis or the discovery of sensitive, early biomarkers of hepatic dysfunction, early portal hypertension, and portal shunt.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.R. and N.S. conceived and designed research; M.R. and N.S. performed experiments; M.R. and N.S. analyzed data; M.R. and N.S. interpreted results of experiments; M.R. and N.S. prepared figures; M.R. and N.S. drafted manuscript; M.R. and N.S. edited and revised manuscript; M.R. and N.S. approved final version of manuscript.
REFERENCES
- 1.Cook Medical . New Portal Pressure Measurement Device Coming to US Physicians. https://www.cookmedical.com/newsroom/new-portal-pressure-measurement-device-coming-to-us-physicians/, 2020.
- 2.Aragonès G, Colom-Pellicer M, Aguilar C, Guiu-Jurado E, Martínez S, Sabench F, Antonio Porras J, Riesco D, Del Castillo D, Richart C, Auguet T. Circulating microbiota-derived metabolites: a “liquid biopsy? Int J Obes, 44: 875–885, 2020. doi: 10.1038/s41366-019-0430-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baffy G. MicroRNAs in nonalcoholic fatty liver disease. J Clin Med 4: 1977–1988, 2015. doi: 10.3390/jcm4121953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bazarbashi AN, Wang TJ, Thompson CC, Ryou M. Endoscopic ultrasound-guided treatment of gastric varices with coil embolization and absorbable hemostatic gelatin sponge: a novel alternative to cyanoacrylate. Endosc Int Open 8: E221–E227, 2020. doi: 10.1055/a-1027-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, Waxman I. Acquisition of portal venous circulating tumor cells from patients with pancreaticobiliary cancers by endoscopic ultrasound. Gastroenterology 149: 1794–1803.e4, 2015. doi: 10.1053/j.gastro.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang JY, Samarasena JB, Tsujino T, Lee J, Hu KQ, McLaren CE, Chen WP, Chang KJ. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. Gastrointest Endosc 85: 996–1001, 2017. doi: 10.1016/j.gie.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu Y, Fang J, Zou L, Cui L, Liang X, Lim SG, Dan YY, Ong CN. Omega-6-derived oxylipin changes in serum of patients with hepatitis B virus-related liver diseases. Metabolomics 14: 26, 2018. doi: 10.1007/s11306-018-1326-z. [DOI] [PubMed] [Google Scholar]
- 8.Mann J, Reeves HL, Feldstein AE. Liquid biopsy for liver diseases. Gut 67: 2204–2212, 2018. doi: 10.1136/gutjnl-2017-315846. [DOI] [PubMed] [Google Scholar]
- 9.Rappaport AM. Hepatic blood flow: morphologic aspects and physiologic regulation. Int Rev Physiol 21: 1–63, 1980. [PubMed] [Google Scholar]
- 10.Soty M, Gautier-Stein A, Rajas F, Mithieux G. Gut-brain glucose signaling in energy homeostasis. Cell Metab 25: 1231–1242, 2017. doi: 10.1016/j.cmet.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 11.Suciu AM, Crisan DA, Procopet BD, Radu CI, Socaciu C, Tantau MV, Stefanescu HO, Grigorescu M. What’s in metabolomics for alcoholic liver disease? J Gastrointestin Liver Dis 27: 51–58, 2018. doi: 10.15403/jgld.2014.1121.271.ald. [DOI] [PubMed] [Google Scholar]
- 12.Trikudanathan G, Pannala R, Bhutani MS, Melson J, Navaneethan U, Parsi MA, Thosani N, Trindade AJ, Watson RR, Maple JT; ASGE Technology Committee . EUS-guided portal vein interventions. Gastrointest Endosc 85: 883–888, 2017. [Erratum in: Gastrointest Endosc 85: 1312, 2017.]10.1016/j.gie.2017.02.019. . [DOI] [PubMed] [Google Scholar]
- 13.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 15: 397–411, 2018. [Erratum in: Nat Rev Gastroenterol Hepatol 15: 785, 2018.] doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Kusumanchi P, Ross RA, Heathers L, Chandler K, Oshodi A, Thoudam T, Li F, Wang L, Liangpunsakul S. Serum metabolomic profiling identifies key metabolic signatures associated with pathogenesis of alcoholic liver disease in humans. Hepatol Commun 3: 542–557, 2019. doi: 10.1002/hep4.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]