Abstract
The efficacy of drugs administered by traditional routes is limited by numerous biological barriers that preclude reaching the intended site of action. Further, full body systemic exposure leads to dose-limiting, off-target side effects. Topical formulations may provide more efficacious drug and nucleic acid delivery for diseases and conditions affecting mucosal tissues, but the mucus protecting our epithelial surfaces is a formidable barrier. Here, we describe recent advances in mucus penetrating approaches for drug and nucleic acid delivery to the ocular surface, the female reproductive tract, the gastrointestinal tract, and the airways.
Keywords: mucus, nanomedicine, mucus penetrating, mucoinert, mucoadhesive, drug delivery
Limitations of conventional drug delivery approaches
Therapeutic efficacy is often limited by the lack of selective delivery to the intended site of action. Therapeutics must overcome numerous biological barriers before reaching target cells and tissues [1, 2], particularly when administered by the most common routes. Oral formulations, such as pills, are first exposed to the acidic and degradative gastrointestinal (GI) environment [1, 2]. The fraction that is absorbed through the intestines travels to the liver where drugs and nucleic acids may be metabolized and inactivated prior to elimination [2]. Intravenous injection bypasses the GI tract barriers, though the entire body is exposed via the nearly 100,000 miles of blood vessels in the adult human body [3]. Further, systemic delivery only achieves modest accumulation of therapeutics in privileged sites due to natural transport barriers, such as the blood-aqueous barrier and the blood-retina barrier in the eye [4, 5]. Thus, in order to achieve the therapeutic levels required at a particular location, high doses must be used, resulting in off-target side effects. In addition, rapid clearance may necessitate frequent dosing, further increasing risk of side effects and user non-adherence. Engineering of formulations for local drug and nucleic acid administration has the potential to overcome these challenges, increase patient compliance, and improve therapeutic efficacy.
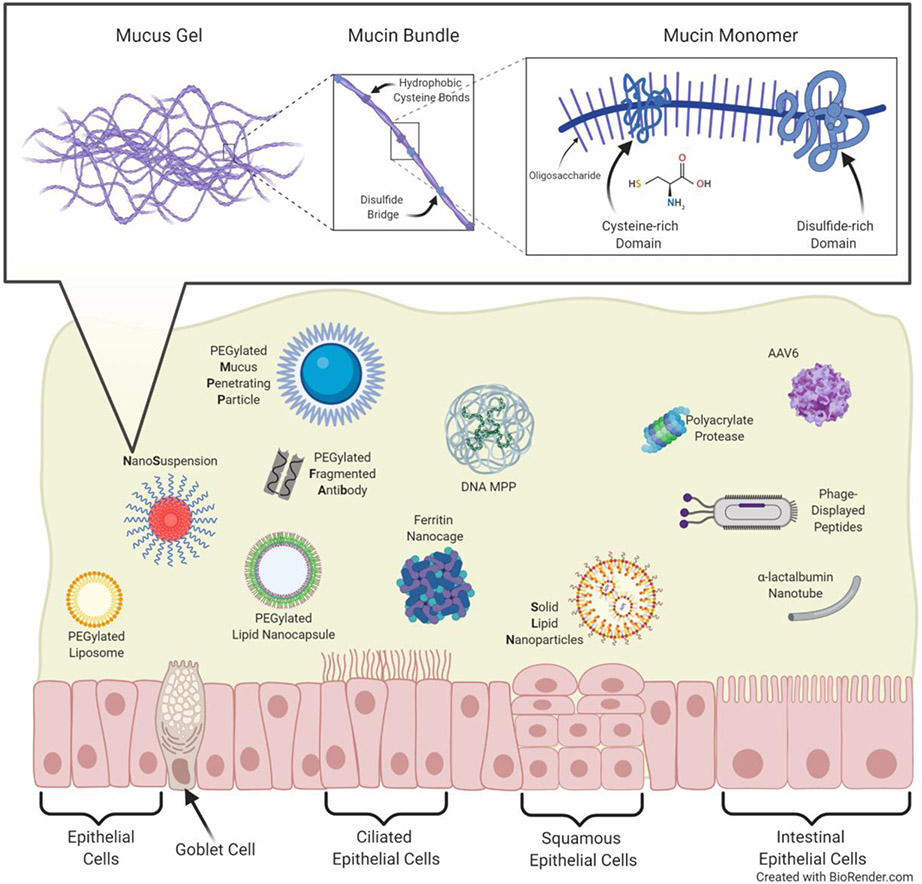
Locally delivered therapies may provide increased local concentrations and fewer off-target side effects [1, 6, 7]. However, when considering local delivery to the ocular, vaginal, rectal, and respiratory surfaces, formulations must be engineered to overcome the protective mucus coating. Mucus is a complex mixture of mucin proteins, ions, lipids, cells, and bacteria [8]. Mucin proteins secreted by goblet cells (see Glossary) form a web-like network via disulfide bridges, calcium crosslinks, and hydrogen bonding interactions (Figure 1, Key Figure). The resulting pore structure traps foreign particulates and pathogens sterically, while the negatively charged glycans and hydrophobic regions further add adhesive barrier properties [8, 9]. Furthermore, mucus is continually secreted and cleared from the body, taking any trapped material with it [8]. While mucus protects the underlying epithelial surfaces from pathogen invasion, it also functions as a barrier to locally administered therapies. Since 2007, mucus penetrating particle (MPP) technology has been utilized to improve the local delivery of drugs and nucleic acids to a variety of mucosal surfaces [10-16]. In addition to mucus penetrating approaches, we review temporary mucus-disrupting techniques that have been utilized to bypass the mucus barrier. The local delivery approaches covered here have been shown to enhance biodistribution, increase cargo (drug or nucleic acid) uptake, and improve disease outcomes in a variety of preclinical models. Importantly, technologies covered in this review has been developed and commercialized into FDA-approved products, demonstrating their clinical relevance (see Clinician’s Corner). We also emphasize challenges and future considerations associated with local mucosal drug delivery, in an effort to encourage continued efforts in the area.
Figure 1 (Key Figure): Mucus and Mucus Penetrating Delivery Systems.
Mucus protects our epithelial layers from foreign pathogens and particulates. The mesh-like pore structure traps by both steric and adhesive interactions. Several mucus penetrating drug and nucleic acid delivery systems described herein are depicted.
Clinician’s Corner.
Why is it that many drugs require frequent dosing and have dose limiting side effects? The ways we typically dose drugs (oral, intravenous) results in full body exposure while only small amounts reach target cells and tissues. To put it another way, most drugs have a delivery problem.
Local administration to mucosal surfaces can provide more targeted delivery of higher levels of drugs and nucleic acids for the treatment and prevention of a wide variety of diseases and conditions. However, effectively delivering drugs and nucleic acids to mucosal tissues is limited by the barrier function of the mucus coating and protecting our epithelial surfaces.
Engineering formulations to overcome the mucus barrier has led to improved treatment of diseases affecting the eye, the female reproductive tract, the gastrointestinal tract, and the airways in a variety of preclinical models [14, 16, 20, 103]. Importantly, the mucus penetrating particle (MPP) approach has been successfully translated from the bench to the clinic in the form of eye drops.
Eye drops are a mainstay for ocular drug delivery, although only a small fraction of topically administered drugs are absorbed into the eye prior to clearance by reflexive tearing and blinking. Recently, the MPP platform has been utilized to improve the intraocular penetration of poorly water-soluble drugs, such as the steroid loteprednol etabonate [58, 62]. In 2018, an MPP formulation was approved for treatment of post-cataract inflammation and pain with twice daily dosing, when the comparator requires four times daily dosing. In October 2020, a second new eye drop drug was approved as the first-in-class temporary treatment of the signs and symptoms of dry eye disease.
The question now is, what is next? There are many other target indications for treatment of ocular diseases, including those affecting the posterior segment, and we have yet to see product development for other mucosal sites. Preclinical data suggests that improved treatments for obstructive airway diseases, inflammatory bowel diseases, and a variety of women’s health indications could be next in the pipeline.
Engineering formulations to overcome mucus barriers
Physical characteristics of mucus penetrating particles
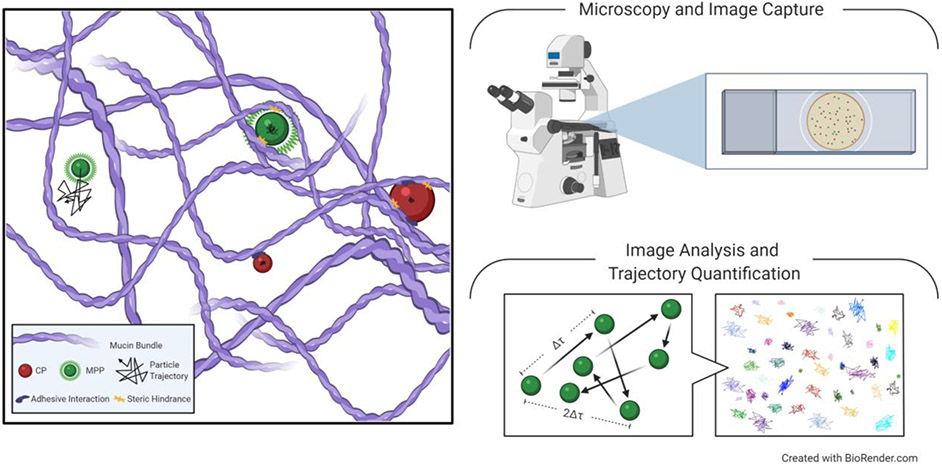
To engineer formulations for enhanced mucosal drug and nucleic acid delivery, we must first better understand mucus structure and barrier function. Both ex vivo and in vivo techniques have been used to probe the interactions between nanoparticles and mucus. Using multiple particle tracking (MPT), it has been shown in multiple contexts that uncoated conventional particles (CPs) adhesively interact with both healthy and diseased mucus layers (Figure 2) [17-19]. Importantly, the hindrance of CP mobility observed in mucus ex vivo corresponds with inadequate distribution of nanoparticles in vivo [20]. By densely coating CPs with hydrophilic polyethylene glycol (PEG)-containing polymers, particle surfaces are shielded from adhesive interactions with mucin proteins [7, 20, 21]. The resulting mucus penetrating particles (MPPs) are hindered only by the pore size of mucus, thus allowing the characterization of the heterogeneous mucus pore structure. For example, it was previously estimated that the pores in cervicovaginal mucus (CVM) were ~100 nm in size [22]. However, using fluorescently labeled MPPs as a probe, it was estimated the average pore size in CVM to be 340 ± 70 nm, with a range of 50-1800 nm [11]. The previous underestimate was likely due to adhesive interactions between CPs and the mucus mesh [22]. MPT using non-adhesive MPPs as probes has revealed that much larger particles can be utilized for effective mucosal drug delivery at a variety of mucosal sites, though the upper size limit depends on the mucus type and the disease state [11, 14, 16, 20, 23].
Figure 2: Particle Mobility in Mucus.
Multiple Particle Tracking (MPT) is used to understand particle mobility in mucus by tracking and quantifying particle trajectory through a biological sample. Conventional particles (CPs) interact with charged and hydrophobic regions of mucin proteins, resulting in physical entrapment. Mucus penetrating particles (MPPs) are coated to avoid adhesive interactions with mucus, allowing for unrestricted Brownian motion through the mucus mesh, but can still be trapped via steric hindrance. Using MPT to track multiple sizes of MPPs allows for characterization of the size and distribution of pores in the mucus mesh. Additionally, quantitative results from MPT can be correlated with particle distribution in vivo.
In addition to being small enough to fit through the pores in the mucus mesh, NPs must also be adequately shielded from adhesive interactions [7, 8, 11, 21]. Hydrophilic, net-neutral polymer coatings are typically used to shield particles from interacting with mucin proteins [7, 24]. PEGylated particles are not always mucus penetrating, however. The surface density of the coating is key to shielding the particle surface from adhesive interactions [10, 11]. Early work reported that 100 nm particles moved more slowly in mucus than 200 nm particles [10], though later it was confirmed that this was likely due to insufficient PEGylation of the 100 nm particles to prevent interactions with mucus [11]. Researchers found that by increasing PEG content in blends of poly(lactic-co-glycolic acid) (PLGA)-PEG and PLGA coated particles led to improved NP stability in the presence of mucins in vitro, increased the rate of diffusion in mucus ex vivo, and enhanced vaginal distribution in mice in vivo [21]. PEG molecular weight (MW) is also intertwined with surface density in governing mucus interactions [25]. Recent work demonstrated that with sufficiently high surface density, PEG as large as 40 kDa in MW could be employed to make MPP [25].
In addition to particle size and surface coating, the shape of a particle impacts its diffusivity through mucus [26]. One study compared spherical “big” (200-300 nm) and “small” nanospheres (20-30 nm) to α-lactalbumin nanotubes (NT) categorized as “long” (20 nm diameter, 800-1200 nm length), “short” (20 nm diameter, 100-200 nm length), and “short/rigid” (cross-linked short NT) for their ability to penetrate intestinal mucus. The tubular peptosomes were formed through a self-assembly of amphiphilic peptides. Using MPT, short nanotubes (SNTs) demonstrated increased Brownian motion compared to the other particle types, indicating that SNTs may be good candidates for locally delivering therapeutics across mucosal barriers (Figure 3) [26]. This study calls for future work to further investigate how shape plays a role in mucus penetrating capabilities (Outstanding Questions).
Figure 3: Multiple Particle Tracking to Evaluate Mobility in Mucus Ex Vivo.
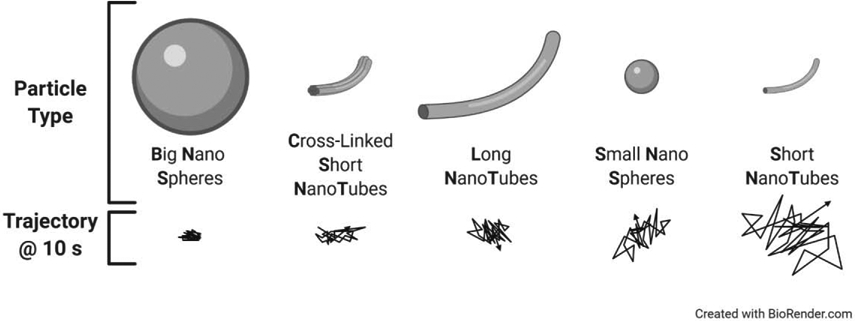
Multiple particle tracking (MPT) was used to evaluate the effect of shape and size on particle mobility in mucus ex vivo. Trajectories for nanospheres and α-lactalbumin nanotubes reveal that short nanotubes (SNTs) showed the highest diffusional mobility in mucus ex vivo. Adapted from Bao, et al., Copyright 2020, with permission from American Chemical Society [26].
Outstanding Questions.
Since the first description of mucus penetrating particles (MPP) in 2007, many different types of MPP systems have been designed and tested in preclinical models. With the rigorous manufacturing, safety, reproducibility, and stability requirements for clinical translation, which of these systems will be next for translation?
Will MPP prove beneficial for treating ocular diseases affecting the posterior segment? What disease indication will be the next clinical targets for MPP-based product development?
Can nanoparticle shape be utilized to further improve mucus penetrating capabilities?
Will PEG immunogenicity play a role with repetitive exposure at mucosal surfaces? In these cases, which PEG alternatives will be most appropriate for clinical development?
In the context of nucleic acid delivery, what additional strategies can be employed that facilitate penetration through mucus barriers without reducing cell uptake efficiency?
What role does the local microbiome play in mucus barrier homeostasis, nanoparticle distribution, and drug uptake, both in health and disease?
Formulation techniques for creating mucus penetrating particles
One commonly used formulation for drug delivery across mucosal surfaces, especially for hydrophobic drugs, is mucoinert nanosuspensions (NS) [13, 14, 27, 28]. NS are particles composed largely of pure drug, which allows for higher drug loading [27]. NS can be formulated using a variety of different “top down” and “bottom up” techniques [27, 29-32]. Wet milling is a top down process that involves the mechanical grinding of hydrophobic drug in the presence of a stabilizer [33]. The grinding beads break apart the drug, and the drug particulates are stabilized by polymers added in the wet milling process [27, 30]. In several studies poloxamer triblock copolymers have been used as stabilizing coatings for hydrophobic drug NS [13]. The general structure of poloxamers is PEG-poly(propylene oxide)-PEG (PEG-PPO-PEG), and they are available in a variety of PEG and PPO MW combinations. NS were formulated with various poloxamers, and it was found that a minimum PPO MW was required to stably adsorb onto the hydrophobic particle surface [13]. Sufficient adsorption of the hydrophobic PPO core resulted in a hydrophilic PEG coating that prevented interaction with mucus [13]. Fluorescent drug NS coated with poloxamer 407 were shown to have high diffusive mobility in both CVM and cystic fibrosis sputum (CFS), leading to significantly increased particle coverage in murine trachea tissue compared to the uncoated NS [13].
Alternatives to PEGylation
Many groups have used PEGylation for shielding particles from adhesive interactions with mucus [7, 20, 21]. However, this approach is not without its limitations. For example, repeated systemic administration of PEGylated particles has been shown to result in production of neutralizing antibodies [34], which may also be possible after repeated mucosal administration [35]. Furthermore, PEGylation provides no advantage to increasing cellular uptake, and the addition of targeting ligands can decrease mucus penetrating properties, thus, alternative strategies have also been proposed for enhancing local drug delivery [24]. Poly(vinyl alcohol) (PVA) is a commonly used excipient, but was described as mucoadhesive when coated onto nanoparticles [20, 36-39]. However, by tuning the degree of PVA hydrolysis, researchers can alter the polymer’s interactions with mucus [40]. Carboxylated polystyrene NPs coated with 75-94% hydrolyzed PVA had increased mobility in human mucus [40]. Similarly, poly(2-alkyl-2-oxazolines) (POZs) have been shown to increase particle mucus penetrating capabilities [41]. POZs structures are easily tunable, which allowed researchers to demonstrate that poly(2-methyl-2-oxazolines) (PMOZs) showed improved mucus penetration compared to poly(2-ethyl-2-oxazolines) or poly(2-propyl-2-oxazolines) [41]. Systemic exposure to poly-(N-(2-hydroxypropyl)methacrylamide) (PHPMA), another hydrophilic polymer used to create mucus penetrating coatings, did not result in the immune response often observed from repetitive PEG exposure [42]. Polyzwitterionic polymer coatings resulted in NPs with equivalent mucus penetration and enhanced cellular uptake, as compared to PEGylated NPs [43]. An expanded toolbox of materials that can be used for mucus penetration will facilitate formulation development for a wider range of therapeutic applications.
As several coatings have been shown to be mucoinert, a recent study compared poloxamer 407, Tween 80, and PVA coatings on solid lipid nanoparticles (SLNs). Single particle tracking (SPT) and time-lapse confocal imaging revealed that poloxamer 407-coated SLNs showed the most rapid diffusion through artificial sputum and CF sputum [44]. It should be noted, that when comparing particle diffusivities, it is important to account for differences in particle size, shape, and experimental techniques, leaving room for more comparative studies in the future.
Outside of the realm of polymers, a phage display library was used to identify peptides with reduced interactions with mucus [45]. Phages were screened for enhanced mucus transport in an in vitro system, resulting in the identification of 26 unique peptides with mucus penetrating capabilities [45]. While further work should be done to explore the viability of these phages in vivo, there is certainly the potential for development of translational drug delivery platforms [45].
Another method used to enhance particle penetration through mucus is disrupting the mucus barrier using proteases designed to cleave muco-glycoprotein substructures [46-48]. In a 2017 study, polyacrylate (PAA) was covalently attached to proteases, papain (PAP) or bromelain (BROM) [49]. PAA-PAP microparticles showed increased penetration, as compared to either PAA-BROM or PAA in porcine intestinal mucus ex vivo [49]. In rats, orally administered PAA-PAP microparticles showed increased concentration in the upper small intestine, whereas PAA-BROM microparticles showed increased concentration in the lower small intestine [49]. In a similar approach, N-acetyl-L-cysteine (NAC) has been used as a reducing agent to disrupt disulfide linkages between mucins, leading to increased particle mobility [50]. In one study, pretreatment with NAC significantly improved gene transfection in the murine lung [50]. Another study showed that orally administering a choline and geranate (CAGE) ionic liquid formulation decreased the viscosity of a mucin hydrogel [51]. This formulation enhanced the delivery of insulin through mucolytic behavior while simultaneously inhibiting proteolytic enzyme activity [51]. These strategies may be useful for short-term administration, but potential impairment of the protective function of mucus with repeated long-term dosing warrants investigation. Additional strategies for mucus penetration have been thoroughly reviewed in a recent special issue of Advanced Drug Delivery Reviews [52].
Overcoming the ocular mucus barrier
Delivery to the anterior portion of the eye
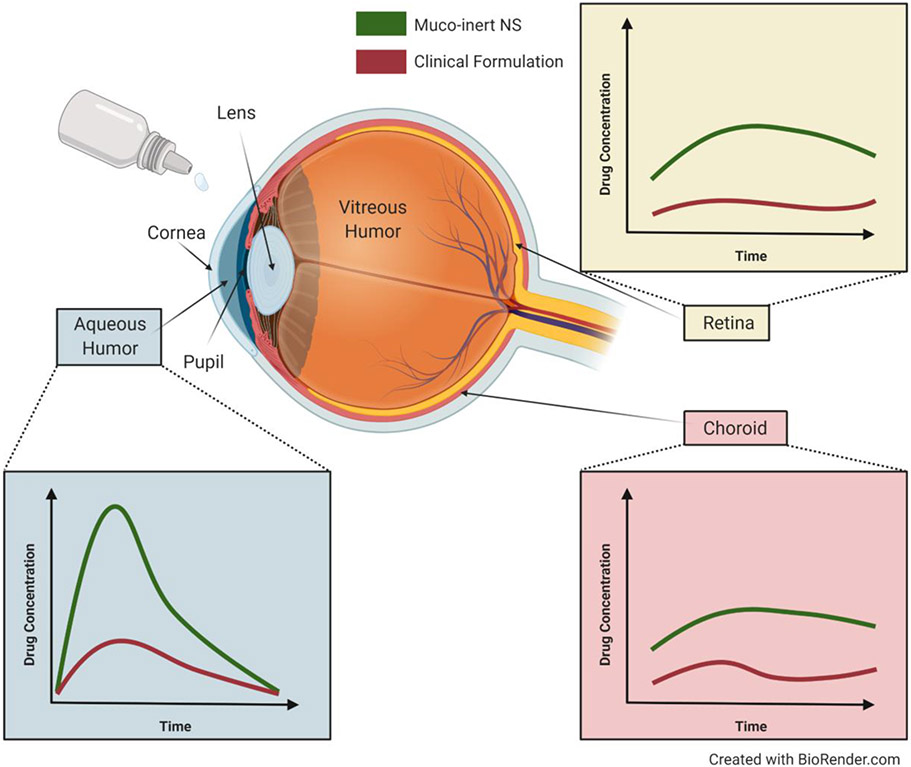
Topical drug administration via eye drops is the mainstay of ocular drug delivery [53, 54]. However, drugs administered in eye drops must penetrate through the mucins in the tear film and the glycocalyx on the tissue surface prior to being blinked away [55, 56]. The average adult blinks once every 4 seconds [57], aiding in the clearance of drugs and drug-loaded particles for treatment of ocular conditions [8, 55]. Recently, the MPP technology has been commercialized for ocular use. Loteprednol etabonate (LE) was formulated as a mucus penetrating drug NS (LE-MPP). Studies in rabbits showed significantly higher LE concentrations in the aqueous humor, cornea, conjunctiva, iris, and central retina when dosed as LE-MPP compared to a commercially available eye drop (Figure 4) [58-60]. In 2018, the FDA approved an LE-MPP product for post-surgical cataract inflammation and pain with half the dosing frequency (2x per day compared to 4x per day) [58, 61]. A second LE-MPP product at a different dosage strength was approved for the short-term treatment of the signs and symptoms of dry eye disease in 2020 [55, 62-64].
Figure 4: Overcoming the Ocular Mucus Barrier.
Schematic of the ocular anatomy with drug concentrations in the aqueous humor (blue box), retina (yellow box), and choroid (red box) after topical administration of muco-inert NS formulation (green line) or clinical formulation (red line). Adapted from Schopf, et al., Copyright 2014, with permission from Springer Nature, and Schopf, et al., Copyright 2015, with permission from ARVO [60, 65].
Delivery to the posterior portion of the eye
Conventionally, eye drops are prescribed for treatment to the anterior portion of the eye. However, preclinical formulations have demonstrated the potential for MPP technology to enhance drug delivery to the posterior eye, specifically for treatment of age-related macular degeneration (AMD) [65]. AMD is the leading cause of blindness in people over the age of 65 [66]. Current treatments for AMD primarily consist of anti-VEGF intravitreal injections, which are uncomfortable and inconvenient for patients, have the added risk of complications, and can occur as often as monthly [67-72]. A novel small molecule receptor tyrosine kinase inhibitor was delivered as a mucoinert NS eye drop, resulting in drug concentrations in the porcine choroid and retina above the IC50 for the drug (Figure 4) [65]. In a separate study, solid lipid nanoparticles (SLNs) were loaded with Atorvastatin for the treatment of AMD [73]. The SLNs were formulated with poloxamer 188 and PEG 400 via a high-pressure homogenization (HPH) technique. Atorvastatin SLNs were ~250 nm in diameter, with a net neutral surface charge [73]. Upon dosing to rabbits, pharmacokinetic analyses revealed that the SLNs delivered significantly more drug to both the aqueous and vitreous humor, as compared to free atorvastatin [73]. These studies indicate the potential for mucoinert eye drop formulations as treatments for AMD.
Overcoming the mucus barrier in the female reproductive tract
Drug delivery to the female reproductive tract
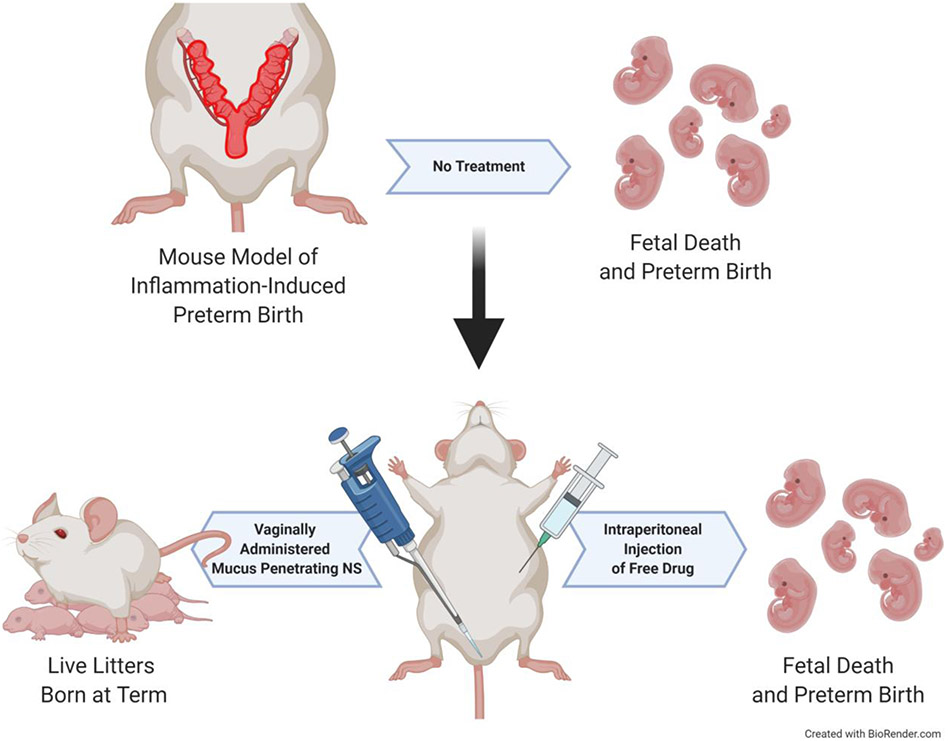
Vaginal drug administration can more effectively target both the lower and upper female reproductive tract (FRT) [74]. The vasculature around the FRT leads to enhanced drug accumulation in the upper tract via the uterine-first-pass effect [74]. This phenomenon, first named in 1997, describes the preferential transport of vaginally administered drugs to the uterus prior to reaching systemic circulation [74]. Indeed, it was shown that vaginally delivered progesterone (P4) led to a 10-fold increase of P4 in the endometrium compared to systemic P4 administration [74]. Vaginal P4 formulations are used clinically in the context of fertility support and preterm birth (PTB) prevention [75-77]. In a preclinical murine model of PTB induced via P4 withdrawal, a mucoinert P4 NS showed improved PTB prophylaxis compared to a clinically used vaginal P4 gel [16]. This PTB prevention was likely due to the enhanced area under the curve (AUC) seen in the P4 NS group, as compared to the P4 gel [16]. In a second study, vaginally delivered mucoinert NS significantly prevented inflammation-induced PTB, whereas systemic administration of the same drug combination did not prevent PTB (Figure 5) [28]. This study specifically highlighted the benefits of effective local drug delivery in understanding drug action in diseased states.
Figure 5: Mucus Penetrating Nanosuspension to Prevent Preterm Birth.
Preterm birth (PTB) was induced in pregnant mice via intrauterine lipopolysaccharide (LPS) injection. Dams receiving no treatment delivered prematurely with no surviving pups. To compare the efficacy of local and systemic drug exposure in preventing PTB, one cohort of dams received a vaginal administration of a mucus penetrating nanosuspension (NS) formulation at the time of PTB induction, and a second cohort received an intraperitoneal (I.P.) injection of free drug. The cohort receiving vaginally administered mucus penetrating NS delivered live pups at term, whereas the cohort receiving I.P. drug delivered prematurely with no surviving offspring. Adapted from Zierden, et al., Copyright 2020, with permission from American Association for the Advancement of Science [28].
In addition to pregnancy-related conditions, vaginal administration is also a potential route for improved prevention of infections of the FRT. PEGylated poly(epsilon-caprolactone) (PCL) particles loaded with dapivirine, an anti-HIV microbicide candidate, were formulated via nanoprecipitation [78]. The resulting NPs were 180-200 nm in size and were able to penetrate the vaginal mucus barrier to reach the epithelial surface in a mouse model [78, 79]. Furthermore, dapivirine was still detected in vaginal lavage fluid 24 h after administration. PK studies revealed that the NP formulation provided higher AUC in the vagina and upper and lower uterus, as compared to free drug in solution, highlighting the benefits of mucus penetrating particles for local vaginal drug delivery [78].
Protein and gene delivery to the female reproductive tract
Building on the principles of drug delivery to the FRT, PEGylated liposomes designed to deliver interferon alfa-2b (INF a-2b) were vaginally administered for the treatment of human papilloma virus (HPV) [80]. In an in vitro study, liposomes were incubated with porcine mucus, and mucin binding was calculated. Significantly less mucin bound to the PEGylated liposomes compared to either non-coated liposomes or mucoadhesive liposomes [80]. Ex vivo studies using vaginal tissue from sheep demonstrated that the PEGylated liposomes provided increased delivery of INF a-2b into vaginal tissue compared to free INF a-2b [80]. Further work should be done to investigate the in vivo performance of these liposomes. MPP approaches have also been employed to enhance nucleic acid delivery across mucus barriers. For example, PEGylated polysuccinimide-based NPs loaded with siRNA were formulated to block viral transmission of HIV and HSV-2 in the FRT [81]. Using an in vitro assay [82], it was shown that twice as much siRNA passed through artificial mucus when loaded into the PEGylated particles compared to free siRNA [81]. While these studies highlight the benefits of overcoming the mucus barrier to enhance therapeutic outcomes for women’s health indications, future work should be done to investigate how hormones, disease, and the local vaginal microbiome may contribute to nanoparticle distribution and uptake in the FRT [83].
Overcoming gastrointestinal mucus barriers
Enemas are used clinically to treat a variety of colorectal diseases and disorders [84]. One such product includes a micronized budesonide enema product used to treat active inflammatory bowel disease (IBD). It was previously reported in a preclinical model of IBD that MPP demonstrated increased colorectal distribution and penetration into inflamed, ulcerated tissue regions [85]. In comparing the clinically used budesonide microsuspension (MS) to a novel mucoinert, poloxamer 407-coated budesonide NS, the NS provided a significant improvement in colon tissue weight and histopathological structure, as well as decreased infiltration of inflammatory macrophages in the colon compared to the budesonide MS [14]. Similarly, model fluorescent particles demonstrated enhanced mucus penetration in mucus ex vivo, corresponding with colorectal distribution in vivo [14]. In another study, coating PLGA NPs with poloxamer 407 significantly increased particle distribution in the colorectum, as compared to non-coated PLGA NPs [86]. Furthermore, PEG-PLGA NPs showed enhanced retention in the colorectum 2 h post-particle administration [86]. Continuing this work, PEG-PLGA NPs were loaded with efavirenz, a non-nucleoside reverse transcriptase inhibitor with antiretroviral activity [87]. While both coated and non-coated NPs improved drug distribution compared to free efavirenz, the PEG-PLGA NPs provided prolonged drug exposure in colon [87]. These studies demonstrate the benefits of using MPPs for colorectal ailments, however future work should be done to investigate how inflammation and disease state contribute to the mucus barrier, and the corresponding design criterion for locally delivered therapies.
Overcoming the mucus barrier in the respiratory tract
Drug delivery to the lung
Obstructive lung diseases, such as chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and asthma, have been shown to benefit from locally delivered therapies [88, 89]. Many studies have investigated mucoadhesive nanoparticles (MAPs) for delivery to the respiratory tract, under the rationale that MAPs adhere to airway mucus, so serve as drug depots for extended release [90, 91]. However, mucociliary clearance in the airways leads to rapid mucus layer turnover, as quickly as 15 min [8]. MPP approaches can improve local drug and nucleic acid delivery to the lung epithelium by penetrating through the superficial layers of airway mucus, reaching the less rapidly cleared periciliary layer [88, 92]. In a model system, fluorescent polystyrene MPPs distributed more uniformly in the airways than their MAP counterparts [92]. In a model of acute lung inflammation, free dexamethasone, dexamethasone loaded MPPs, and dexamethasone loaded MAPs were compared in their ability to reduce inflammatory cell counts in bronchoalveolar lavage fluid (BALF) [92]. While free dexamethasone and poloxamer 188-coated PLGA MAPs only had a modest effect on inflammatory cell counts, poloxamer 407 coated PLGA (PLGA/407) MPPs significantly reduced lung airway inflammation. Furthermore, only treatment with PLGA/407 MPPs significantly reduced inflammatory markers in BALF [92]. In another study, PLA-PEG nanoparticles loaded with fluticasone propionate (FP) by a wet milling process [93]. FP was detected in lung tissue for up to 24 h after a single intratracheal administration of FP-loaded MPPs in mice. Following an administration of either free FP or FP MPPs, healthy rats were challenged with lipopolysaccharide (LPS) to model pulmonary inflammation. The rats receiving treatment with FP MPPs showed a significant decrease in neutrophil recruitment in BALF as compared to treatment with free FP [93]. Ciprofloxacin-loaded lipid-core nanocapsules (LNC-CIP) were formulated to prevent Pseudomonas aeruginosa and Staphylococcus aureus infections, which commonly occur in CF patients [94]. The particles were formed using oleic acid and polysorbate 80 in an interfacial deposition of polymer technique, and were <200 nm in diameter. An ex vivo permeation assay revealed that LNC-CIP showed increased penetration through horse lung mucus, as compared to an aqueous drug suspension [94]. Furthermore, the antibacterial activity of ciprofloxacin was not affected due to encapsulation in the LNCs, and LNCs demonstrated the ability to prevent biofilm formation in vitro [94]. Similarly, mucus penetrating SLNs were designed to deliver a quorum sensing inhibitor (QSI) to control P. aeruginosa infections [95]. The SLNs were formulated using a combination of lipids in aqueous solution and a poloxamer 407 coating. Particle size was controlled with a combination of probe sonication and high shear homogenization. In artificial sputum, confocal microscopy confirmed SLNs diffusion through mucus [95]. In vitro, QSI loaded SLNs efficiently inhibited pyocyanin, a molecule that was shown to be critical for quorum sensing, and thereby virulence control in P. aeruginosa, as compared to free QSI [95]. A separate study compared similarly sized (~50 nm) small unilamellar liposomes, poloxamer 407-coated liposomes, and PEG lipid (PEG 2000PE)-coated liposomes for local beclomethasone dipropionate delivery to the lung [96]. In COPD sputum samples, PEG 2000PE coated liposomes demonstrated improved penetration compared to poloxamer 407 coated liposomes and uncoated liposomes [96]. Comparing PEGylation of antibody fragments (Fab) for treatment of asthma, there were no significant differences between linear 20 kDa PEG-maleimide and two-armed 40 kDa PEG-maleimide in regards to Fab mobility in healthy human respiratory mucus [97]. Furthermore, both PEGylated Fabs showed significantly increased antibody concentration in bronchoalveolar lavage and lung tissue homogenate, as compared to a subcutaneous injection of antibody fragments [97]. Ferritin nanocages (FTn) have been recently described for local respiratory delivery [98]. Recombinant FTn were purified from bacterial cells transformed with a plasmid for expressing human native ferritin heavy chain. The nanocages were PEGylated with various MW PEG (2-10 kDa). It was observed that PEGylation significantly increased epithelial distribution in the murine airway, as compared to non-coated FTn, and a 2 kDa PEG coating led to increased FTn retention in the upper respiratory tract. Doxorubicin was used to test the therapeutic benefits of the FTn in an orthotopic mouse lung cancer model [99]. Mice treated with the doxorubicin-loaded PEGylated FTn showed significantly improved survival compared to mice treated with free doxorubicin, highlighting another potential therapeutic target for the MPP technology [99].
Gene delivery to the lung
Mucus in the airways is also a formidable barrier to effective gene therapy, which requires particles both pass through the mucus barrier and be taken up into cells [88, 89]. CF is an inherited disease caused by a mutation in the CF transmembrane conductance regulator (CFTR) gene that leads to a significant increase in airway mucus viscoelasticity [100]. The barrier properties of CF sputum likely play a significant role in the >30 unsuccessful gene therapy trials for CF treatment. In 2014, polyethylenimine (PEI) and poly-L-lysine (PLL) PEGylated particles were developed for gene delivery to the lung [101]. Using a drop-wise approach, DNA plasmids were compacted with polymer blends to form DNA NPs. DNA NPs formed from high percentage (75%) PEG polymer blends showed improved stability and increased diffusional mobility in human CF sputum ex vivo [101]. Intranasal dosing to mice revealed that DNA MPPs were better retained in the airways and provided a significant increase in cell transfection [101]. More recently, DNA MPPs were designed to enhance expression of fibroblast growth factor 2 (FGF2) in the murine respiratory tract [102]. PEGylated DNA MPPs demonstrated increased diffusional mobility in human CF sputum ex vivo, leading to increased distribution of particles in the mouse airways in vivo. Quantification via MPT revealed that 40% particle PEGylation led to the most rapid diffusion through CF sputum [102]. In a separate study, adeno-associated virus (AAV) were explored for gene delivery in the context of CF [103]. Previous studies had demonstrated that commonly used viral vectors were adhesive to CF sputum, which would prevent effective uptake into epithelial [104]. However, AAV6 was shown to be mucoinert as a result of a mutation in the viral capsid. AAV6 was found to have increased diffusional mobility in CF sputum, and provided a significant increase in gene transfection and model protein expression in a mouse model of obstructive lung disease [103]. Taken together, these studies highlight the utility of MPPs as a mode of drug and nucleic acid delivery to the airways.
Concluding Remarks
Local drug delivery increases therapeutic concentrations in target tissues, while decreasing off-target side effects. For mucosal surfaces, including the eye, female reproductive tract, gastrointestinal tract, and airways, the full benefits of local delivery may only be realized when therapies are rationally engineered to bypass the mucus barrier. For many years, mucoadhesive strategies were utilized in an effort to enhance formulation retention at mucosal sites. In 2007, this longstanding paradigm shifted to using mucus penetrating particle formulations, which have been shown to improve drug and nucleic acid pharmacodynamics, leading to enhanced prevention and treatment of disease. The mucus penetrating particle approach has been translated into FDA-approved therapies for ocular conditions, and is likely to be applied to an array of diseases affecting other mucosal sites (see Outstanding Questions). Continued development of the numerous delivery platforms engineered to overcome the mucus barrier to locally deliver drug and nucleic acid therapies will undoubtedly lead to further improvement in patient care and outcomes.
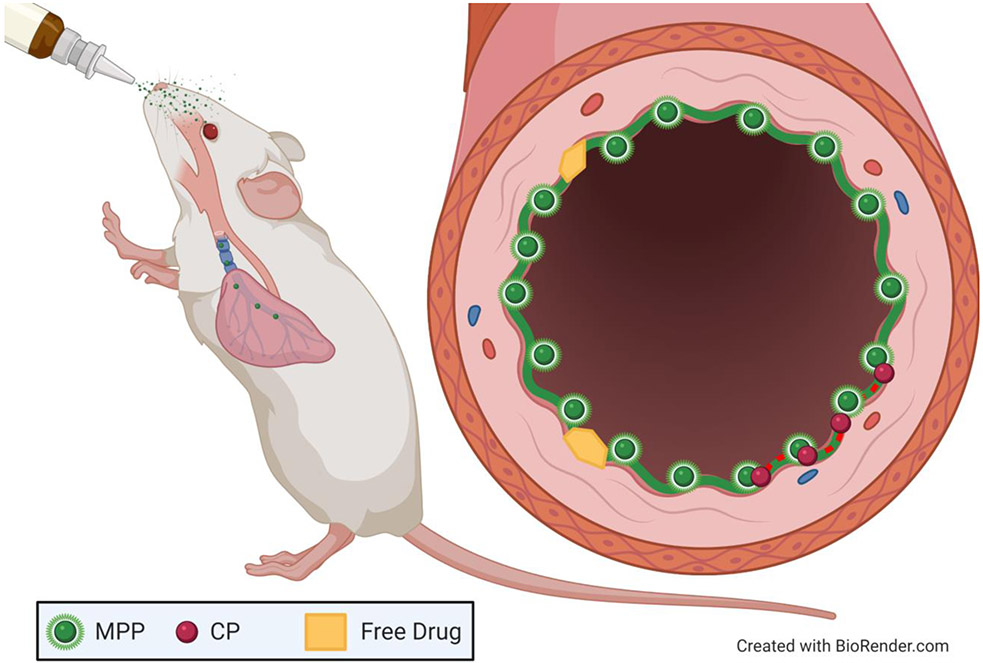
Figure 6: Intranasal Administration of Mucus Penetrating Particles.
Intranasal drug administration is often used for delivery to the respiratory tract. In several studies, mucus penetrating particles (MPPs), carrying drug or genetic cargo, have been shown to increase coverage in the airways of rodents, as compared to conventional particles (CPs) or free drug.
Table 1:
Delivery systems for targeting the ocular surface, respiratory tract, gastrointestinal (GI) tract, and female reproductive tract (FRT).
| Delivery System | Demonstrated Applications |
Benefits | Challenges | Ref. |
|---|---|---|---|---|
| α-lactalbumin Nanotubes | GI tract – Short nanotubes show highest diffusive mobility of all shapes in study | High biocompatibility; Easily tunable | Difficult to conjugate/encapsulate drug; No evidence of mucus penetration in vivo | [26] |
| Adeno-Associated Virus (AAV) | Respiratory tract – AAV6 shown to be mucoinert in cystic fibrosis (CF) sputum, increased gene delivery and protein expression in mice | Targeted; Easily manipulated; Escapes enzymatic degradation | Innate or generated immunogenicity; Further efforts needed to understand safety and efficacy | [103] |
| Fragmented antibodies (Fab) | Respiratory tract – PEGylated Fab penetrate respiratory mucus for the treatment of asthma | Improved mucus penetration and residence time of antibody in vivo; Demonstrated superiority to subcutaneous injection of antibody | Antibodies can be altered and/or knocked down by the native immune system; Not all antibody treatments are effective in every patient | [97] |
| Nucleic acid delivery systems | Respiratory tract – therapeutic gene replacement in CF; FRT – prevention of HIV and HSV-2 transmission via siRNA delivery | Alters genetic makeup to fix/prevent disease at its genetic core instead of masking/treating symptoms | Difficult to maintain balance between particle stability and mucus penetration while maintaining high cellular uptake | [81, 82, 88, 89, 101, 102] |
| Liposomes | FRT – inf-alpha-2B to treat HPV; Respiratory tract – beclomethasone dipropionate for treatment of chronic obstructive pulmonary disease (COPD) | Versatile platform; Can encapsulate both hydrophobic and hydrophilic drugs; Size can be easily manipulated | Expensive and complicated to produce on a large scale; Particles are relatively unstable at long timescales | [80, 96] |
| Nanosuspension (NS) | Examples of NS enhancing delivery to the ocular surface, FRT, GI tract, and respiratory tract; FDA approved formulation for post-cataract surgery inflammation and pain | Can use FDA approved stabilizers; Successfully translated into approved products; Increased drugloading | Less amenable to formulation using water soluble drugs; The addition of stabilizers may add variability and impact tolerability/toxicity | [13, 14, 16, 27, 29, 30, 55] |
| Phage-Displayed Peptides | Tested in CF mucus model | Few off-target side effects; Easily manipulated | Viability of peptides/phages in vivo has yet to be explored | [45] |
| Proteases | Respiratory tract/GI tract—Proteases cleave muco-glycoprotein substructures, reducing mucus mechanical integrity and barrier properties | Versatile; May not require alterations to delivery vehicle | Alters mechanical integrity of mucus, potential concerns with long-term dosing | [46-49] |
Highlights.
Local drug and nucleic acid delivery allows for increased concentrations in target cells and tissues with decreased off-target side effects.
Local drug and nucleic acid delivery to mucosal tissues is limited by the mucus layers coating and protecting the underlying epithelial surfaces; formulations must be engineered to penetrate the mucus barrier for maximal delivery benefit.
Recent work applying local delivery strategies that overcome the mucus barrier has highlighted improved treatment and prevention of diseases affecting the eye, the female reproductive tract, the gastrointestinal tract, and the airways.
Acknowledgments
The work was supported by the Burroughs Wellcome Preterm Birth Initiative, grant 1015020 awarded to L.M.E., the National Institutes of Health (NIH) (R01DK107806, R01EY026578, R01EY031041, R01HL136617, U19AI113127), the Robert H. Smith Family Foundation, and a departmental grant from Research to Prevent Blindness. H.C.Z. was supported by an NSF GRFP Fellowship (DGE-1746891). BioRender was used to create all figures. All authors contributed to the manuscript, assisted in revisions, read and approved the submitted version.
Glossary
- α-lactalbumin
whey protein found in the milk of most mammalian species.
- Adeno-associated virus (AAV)
small, replication-defective, non-enveloped viruses commonly used as gene therapy vectors due to their mild immunogenicity and ability to infect both dividing and quiescent cells.
- Area under the curve (AUC)
measure of the total drug/nucleic acid exposure in a given physiological compartment over time.
- Brownian motion
Nanoparticles diffuse in solution due to thermal energy. If this diffusional motion is unobstructed by the surrounding environment, the path of the motion is random and referred to as “Brownian”.
- Calcium crosslinks
non-covalent calcium ion bridges between mucin fibers. Ca2+ can form two bonds with negatively charged glycans on mucin proteins, creating the pore structure of mucus that results in steric hindrance of large particles.
- Conventional particles (CPs)
particles which are not coated to avoid adhesive interactions with mucin proteins, sometimes ‘muco-adhesive particles’ (MAPs).
- Disulfide bridges
bonds formed between the thiol (-SH) groups present in cysteine-rich domains. Disulfide rich domains exist at the end of each mucin monomer where ‘bridges’ are linkages between carboxyl-carboxyl ends, and amino-amino ends of mucin monomers to form secondary and tertiary mucus structure. Plays a role in forming the pore structure of mucus.
- Ferritin nanocages (FTn)
non-immunogenic, self-assembled structures comprised of heavy-chain human ferritin, the protein responsible for iron storage and transport.
- Goblet cells
cells that secrete mucins.
- Hydrogen bonding
weak, non-covalent bond between particular hydrogen-containing functional groups, in mucins, interactions largely driven by glycosylation (sugar moieties) on mucins.
- Hydrolyzed
process by which polymer is broken down into monomers in the presence of water.
- Molecular weight
the mass of one mole of a given substance.
- Mucociliary clearance
self-clearance facilitated by cilia (hair-like structures on the surface of cells in) present on epithelial cells in the respiratory tract.
- Mucus penetrating particle (MPP)
particles coated (by various methods, but often times by PEGylation) to avoid adhesive interactions with mucin proteins. Additionally, MPPs should be small enough to fit through mucus pores to avoid steric hindrance by mucus.
- Multiple particle tracking (MPT)
a technique that uses the motion of fluorescent probe nanoparticles to infer structural and adhesive properties of the surrounding environment. For this application, nanoparticles are added fresh, undiluted mucus samples, and particle trajectories are tracked over time. Using mucoinert nanoparticles of various sizes can allow for characterization of the mucus pore sizes.
- Nanosuspensions (NS)
a colloidal dispersion of stabilized, nano-sized drug particles. NS can be formulated using top-down (large drug particles broken down into smaller particles) or bottom-up (solubilized drug is precipitated to form nanoparticles) approaches.
- PEGylated
a descriptor indicating covalent, or non-covalent, coating with polyethylene glycol.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
The mucus-penetrating particle technology is licensed and in clinical development for ocular indications by Kala Pharmaceuticals. J.H. is a founder of Kala Pharmaceuticals and serves as a consultant. L.M.E, J.H., and Johns Hopkins own company stock. Under a licensing agreement between Kala Pharmaceuticals and the Johns Hopkins University, L.M.E., J.H., and the University are entitled to royalty distributions related to the technology. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
References
- 1.Ensign LM et al. (2014) Nanoparticle-based drug delivery to the vagina: a review. J Control Release 190, 500–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ensign LM et al. (2012) Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev 64 (6), 557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aird WC (2005) Spatial and temporal dynamics of the endothelium. J Thromb Haemost 3 (7), 1392–406. [DOI] [PubMed] [Google Scholar]
- 4.Coca-Prados M (2014) The blood-aqueous barrier in health and disease. J Glaucoma 23 (8 Suppl 1), S36–8. [DOI] [PubMed] [Google Scholar]
- 5.Cunha-Vaz J et al. (2011) Blood-retinal barrier. Eur J Ophthalmol 21 Suppl 6, S3–9. [DOI] [PubMed] [Google Scholar]
- 6.Gote V et al. (2019) Ocular Drug Delivery: Present Innovations and Future Challenges. J Pharmacol Exp Ther 370 (3), 602–624. [DOI] [PubMed] [Google Scholar]
- 7.Suk JS et al. (2016) PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 99 (Pt A), 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cone RA (2009) Barrier properties of mucus. Adv Drug Deliv Rev 61 (2), 75–85. [DOI] [PubMed] [Google Scholar]
- 9.Bansil R and Turner BS (2018) The biology of mucus: Composition, synthesis and organization. Adv Drug Deliv Rev 124, 3–15. [DOI] [PubMed] [Google Scholar]
- 10.Lai SK et al. (2007) Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci U S A 104 (5), 1482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai SK et al. (2010) Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc Natl Acad Sci U S A 107 (2), 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensign LM et al. (2013) Enhanced vaginal drug delivery through the use of hypotonic formulations that induce fluid uptake. Biomaterials 34 (28), 6922–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu T et al. (2016) Mucus-Penetrating Nanosuspensions for Enhanced Delivery of Poorly Soluble Drugs to Mucosal Surfaces. Adv Healthc Mater 5 (21), 2745–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Date AA et al. (2018) Mucus-penetrating budesonide nanosuspension enema for local treatment of inflammatory bowel disease. Biomaterials 185, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao P et al. (2018) Hypo-osmolar Formulation of Tenofovir (TFV) Enema Promotes Uptake and Metabolism of TFV in Tissues, Leading to Prevention of SHIV/SIV Infection. Antimicrob Agents Chemother 62 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang T et al. (2019) Development of a mucoinert progesterone nanosuspension for safer and more effective prevention of preterm birth. J Control Release 295, 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster BS et al. (2015) Particle tracking in drug and gene delivery research: State-of-the-art applications and methods. Adv Drug Deliv Rev 91, 70–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim AJ and Hanes J (2012) The emergence of multiple particle tracking in intracellular trafficking of nanomedicines. Biophys Rev 4 (2), 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suh J et al. (2005) Real-time multiple-particle tracking: applications to drug and gene delivery. Adv Drug Deliv Rev 57 (1), 63–78. [DOI] [PubMed] [Google Scholar]
- 20.Ensign LM et al. (2012) Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med 4 (138), 138ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Q et al. (2015) Impact of Surface Polyethylene Glycol (PEG) Density on Biodegradable Nanoparticle Transport in Mucus ex Vivo and Distribution in Vivo. ACS Nano 9 (9), 9217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olmsted SS et al. (2001) Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J 81 (4), 1930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chisholm JF et al. (2019) Nanoparticle diffusion in spontaneously expectorated sputum as a biophysical tool to probe disease severity in COPD. Eur Respir J 54 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khutoryanskiy VV (2018) Beyond PEGylation: Alternative surface-modification of nanoparticles with mucus-inert biomaterials. Adv Drug Deliv Rev 124, 140–149. [DOI] [PubMed] [Google Scholar]
- 25.Maisel K et al. (2016) Nanoparticles coated with high molecular weight PEG penetrate mucus and provide uniform vaginal and colorectal distribution in vivo. Nanomedicine (Lond) 11 (11), 1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao C et al. (2020) Enhanced Transport of Shape and Rigidity-Tuned alpha-Lactalbumin Nanotubes across Intestinal Mucus and Cellular Barriers. Nano Lett 20 (2), 1352–1361. [DOI] [PubMed] [Google Scholar]
- 27.Rabinow BE (2004) Nanosuspensions in drug delivery. Nat Rev Drug Discov 3 (9), 785–96. [DOI] [PubMed] [Google Scholar]
- 28.Zierden H et al. (accepted) Enhanced drug delivery to the reproductive tract using nanomedicine reveals therapeutic options for prevention of preterm birth. Sci Transl Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lestari M et al. (2019) The Scalability of Wet Ball Milling for The Production of Nanosuspensions. Pharm Nanotechnol 7 (2), 147–161. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y et al. (2013) Stability of nanosuspensions in drug delivery. J Control Release 172 (3), 1126–41. [DOI] [PubMed] [Google Scholar]
- 31.Jacob S et al. (2020) Emerging role of nanosuspensions in drug delivery systems. Biomater Res 24, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bommana MM et al. (2019) Chapter 2 - Nanostructures in pharma: elixir to oral medicine. In Nanoparticles in Pharmacotherapy (Grumezescu AM ed), pp. 23–44, William Andrew Publishing. [Google Scholar]
- 33.Peltonen L and Hirvonen J (2010) Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol 62 (11), 1569–79. [DOI] [PubMed] [Google Scholar]
- 34.Park K (2018) Impact of anti-PEG antibodies on PEGylated nanoparticles fate in vivo. J Control Release 287, 257. [DOI] [PubMed] [Google Scholar]
- 35.Henry CE et al. (2016) Anti-PEG antibodies alter the mobility and biodistribution of densely PEGylated nanoparticles in mucus. Acta Biomater 43, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mongia NK et al. (1996) Mucoadhesive poly(vinyl alcohol) hydrogels produced by freezing/thawing processes: applications in the development of wound healing systems. J Biomater Sci Polym Ed 7 (12), 1055–64. [DOI] [PubMed] [Google Scholar]
- 37.Salamat-Miller N et al. (2005) The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev 57 (11), 1666–91. [DOI] [PubMed] [Google Scholar]
- 38.Mert O et al. (2012) A poly(ethylene glycol)-based surfactant for formulation of drug-loaded mucus penetrating particles. J Control Release 157 (3), 455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Q et al. (2013) Scalable method to produce biodegradable nanoparticles that rapidly penetrate human mucus. J Control Release 170 (2), 279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popov A et al. (2016) Mucus-penetrating nanoparticles made with "mucoadhesive" poly(vinyl alcohol). Nanomedicine 12 (7), 1863–1871. [DOI] [PubMed] [Google Scholar]
- 41.Mansfield ED et al. (2016) Side chain variations radically alter the diffusion of poly(2-alkyl-2-oxazoline) functionalised nanoparticles through a mucosal barrier. Biomater Sci 4 (9), 1318–27. [DOI] [PubMed] [Google Scholar]
- 42.Du N et al. (2016) Poly(d,l-lactic acid)-block-poly(N-(2-hydroxypropyl)methacrylamide) nanoparticles for overcoming accelerated blood clearance and achieving efficient anti-tumor therapy. Polymer Chemistry 7 (36), 5719–5729. [Google Scholar]
- 43.Shan W et al. (2016) Enhanced Oral Delivery of Protein Drugs Using Zwitterion-Functionalized Nanoparticles to Overcome both the Diffusion and Absorption Barriers. ACS Appl Mater Interfaces 8 (38), 25444–53. [DOI] [PubMed] [Google Scholar]
- 44.Nafee N et al. (2018) Mucus-penetrating solid lipid nanoparticles for the treatment of cystic fibrosis: Proof of concept, challenges and pitfalls. Eur J Pharm Biopharm 124, 125–137. [DOI] [PubMed] [Google Scholar]
- 45.Leal J et al. (2018) Mucus-penetrating phage-displayed peptides for improved transport across a mucus-like model. Int J Pharm 553 (1-2), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Netsomboon K and Bernkop-Schnurch A (2016) Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur J Pharm Biopharm 98, 76–89. [DOI] [PubMed] [Google Scholar]
- 47.Griessinger J et al. (2015) Methods to determine the interactions of micro- and nanoparticles with mucus. Eur J Pharm Biopharm 96, 464–76. [DOI] [PubMed] [Google Scholar]
- 48.Dunnhaupt S et al. (2015) Nano-carrier systems: Strategies to overcome the mucus gel barrier. Eur J Pharm Biopharm 96, 447–53. [DOI] [PubMed] [Google Scholar]
- 49.Mahmood A et al. (2017) Protease-functionalized mucus penetrating microparticles: In-vivo evidence for their potential. Int J Pharm 532 (1), 177–184. [DOI] [PubMed] [Google Scholar]
- 50.Suk JS et al. (2011) N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles. Mol Ther 19 (11), 1981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banerjee A et al. (2018) Ionic liquids for oral insulin delivery. Proc Natl Acad Sci U S A 115 (28), 7296–7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.das Neves J and Sarmento B (2018) Technological strategies to overcome the mucus barrier in mucosal drug delivery. Adv Drug Deliv Rev 124, 1–2. [DOI] [PubMed] [Google Scholar]
- 53.Gaudana R et al. (2009) Recent perspectives in ocular drug delivery. Pharm Res 26 (5), 1197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel A et al. (2013) Ocular drug delivery systems: An overview. World J Pharmacol 2 (2), 47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Popov A (2020) Mucus-Penetrating Particles and the Role of Ocular Mucus as a Barrier to Micro- and Nanosuspensions. J Ocul Pharmacol Ther 36 (6), 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodrigues GA et al. (2018) Topical Drug Delivery to the Posterior Segment of the Eye: Addressing the Challenge of Preclinical to Clinical Translation. Pharm Res 35 (12), 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sforza C et al. (2008) Spontaneous blinking in healthy persons: an optoelectronic study of eyelid motion. Ophthalmic Physiol Opt 28 (4), 345–53. [DOI] [PubMed] [Google Scholar]
- 58.Kim T et al. (2019) Safety and efficacy of twice daily administration of KPI-121 1% for ocular inflammation and pain following cataract surgery. Clin Ophthalmol 13, 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glogowski S et al. (2014) Prolonged exposure to loteprednol etabonate in human tear fluid and rabbit ocular tissues following topical ocular administration of Lotemax gel, 0.5%. J Ocul Pharmacol Ther 30 (1), 66–73. [DOI] [PubMed] [Google Scholar]
- 60.Schopf L et al. (2014) Ocular Pharmacokinetics of a Novel Loteprednol Etabonate 0.4% Ophthalmic Formulation. Ophthalmol Ther 3 (1-2), 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kala Pharmaceuticals, I., Kala Pharmaceuticals Announces FDA Approval of INVELTYS™ for the Treatment of Post-Operative Inflammation and Pain Following Ocular Surgery Business Wire, 2018. [Google Scholar]
- 62.Korenfeld M et al. (2020) Safety of KPI-121 Ophthalmic Suspension 0.25% in Patients With Dry Eye Disease: A Pooled Analysis of 4 Multicenter, Randomized, Vehicle-Controlled Studies. Cornea. [DOI] [PubMed] [Google Scholar]
- 63.Kala Pharmaceuticals, I., Kala Pharmaceuticals Resubmits New Drug Application for EYSUVIS™ for Dry Eye Disease, BioSpace, 2020. [Google Scholar]
- 64.Kala Pharmaceuticals, I., Kala Pharmaceuticals Announces FDA Approval of Eysuvis for the Short-Term Treatment of the Signs and Symptoms of Dry Eye Disease. 2020. [Google Scholar]
- 65.Schopf LR et al. (2015) Topical Ocular Drug Delivery to the Back of the Eye by Mucus-Penetrating Particles. Transl Vis Sci Technol 4 (3), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell P et al. (2018) Age-related macular degeneration. Lancet 392 (10153), 1147–1159. [DOI] [PubMed] [Google Scholar]
- 67.Rosenfeld PJ et al. (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355 (14), 1419–31. [DOI] [PubMed] [Google Scholar]
- 68.Good TJ et al. (2011) Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. British Journal of Ophthalmology 95 (8), 1111–1114. [DOI] [PubMed] [Google Scholar]
- 69.Hoang QV et al. (2012) Effect on intraocular pressure in patients receiving unilateral intravitreal anti-vascular endothelial growth factor injections. Ophthalmology 119 (2), 321–326. [DOI] [PubMed] [Google Scholar]
- 70.Karagiannis DA et al. (2009) Large subretinal haemorrhage following change from intravitreal bevacizumab to ranibizumab. Ophthalmologica 223 (4), 279–282. [DOI] [PubMed] [Google Scholar]
- 71.Meyer CH et al. (2011) Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta ophthalmologica 89 (1), 70–75. [DOI] [PubMed] [Google Scholar]
- 72.Fintak DR et al. (2008) Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 28 (10), 1395–1399. [DOI] [PubMed] [Google Scholar]
- 73.Yadav M et al. (2020) Atorvastatin-loaded solid lipid nanoparticles as eye drops: proposed treatment option for age-related macular degeneration (AMD). Drug Deliv Transl Res 10 (4), 919–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Ziegler D et al. (1997) The first uterine pass effect. Ann N Y Acad Sci 828, 291–9. [DOI] [PubMed] [Google Scholar]
- 75.Yanushpolsky E et al. (2010) Crinone vaginal gel is equally effective and better tolerated than intramuscular progesterone for luteal phase support in in vitro fertilization-embryo transfer cycles: a prospective randomized study. Fertil Steril 94 (7), 2596–9. [DOI] [PubMed] [Google Scholar]
- 76.Kuon RJ et al. (2010) Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle. Am J Obstet Gynecol 202 (5), 455.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krispin E et al. (2018) The association of different progesterone preparations with preterm birth prevention. J Matern Fetal Neonatal Med, 1–6. [DOI] [PubMed] [Google Scholar]
- 78.das Neves J et al. (2014) Biodistribution and pharmacokinetics of dapivirine-loaded nanoparticles after vaginal delivery in mice. Pharm Res 31 (7), 1834–45. [DOI] [PubMed] [Google Scholar]
- 79.das Neves J et al. (2013) In vitro and ex vivo evaluation of polymeric nanoparticles for vaginal and rectal delivery of the anti-HIV drug dapivirine. Mol Pharm 10 (7), 2793–807. [DOI] [PubMed] [Google Scholar]
- 80.Joraholmen MW et al. (2017) PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur J Pharm Biopharm 113, 132–139. [DOI] [PubMed] [Google Scholar]
- 81.Currie S et al. (2020) Mucus-penetrating PEGylated polysuccinimide-based nanocarrier for intravaginal delivery of siRNA battling sexually transmitted infections. Colloids Surf B Biointerfaces 196, 111287. [DOI] [PubMed] [Google Scholar]
- 82.Craparo EF et al. (2016) Pegylated Polyaspartamide-Polylactide-Based Nanoparticles Penetrating Cystic Fibrosis Artificial Mucus. Biomacromolecules 17 (3), 767–77. [DOI] [PubMed] [Google Scholar]
- 83.Hoang T et al. (2020) The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis. PLoS Pathog 16 (1), e1008236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.das Neves J et al. (2010) Nanotechnology-based systems for the treatment and prevention of HIV/AIDS. Adv Drug Deliv Rev 62 (4-5), 458–77. [DOI] [PubMed] [Google Scholar]
- 85.Maisel K et al. (2015) Effect of surface chemistry on nanoparticle interaction with gastrointestinal mucus and distribution in the gastrointestinal tract following oral and rectal administration in the mouse. J Control Release 197, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nunes R et al. (2018) Surface modification with polyethylene glycol enhances colorectal distribution and retention of nanoparticles. Eur J Pharm Biopharm 130, 200–206. [DOI] [PubMed] [Google Scholar]
- 87.Nunes R et al. (2018) Noncovalent PEG Coating of Nanoparticle Drug Carriers Improves the Local Pharmacokinetics of Rectal Anti-HIV Microbicides. ACS Appl Mater Interfaces 10 (41), 34942–34953. [DOI] [PubMed] [Google Scholar]
- 88.Duncan GA et al. (2016) The Mucus Barrier to Inhaled Gene Therapy. Mol Ther 24 (12), 2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim N et al. (2016) Barriers to inhaled gene therapy of obstructive lung diseases: A review. J Control Release 240, 465–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Surendrakumar K et al. (2003) Sustained release of insulin from sodium hyaluronate based dry powder formulations after pulmonary delivery to beagle dogs. J Control Release 91 (3), 385–94. [DOI] [PubMed] [Google Scholar]
- 91.Sakagami M et al. (2002) Mucoadhesive beclomethasone microspheres for powder inhalation: their pharmacokinetics and pharmacodynamics evaluation. J Control Release 80 (1-3), 207–18. [DOI] [PubMed] [Google Scholar]
- 92.Schneider CS et al. (2017) Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci Adv 3 (4), e1601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Popov A et al. (2016) Enhanced pulmonary delivery of fluticasone propionate in rodents by mucus-penetrating nanoparticles. Int J Pharm 502 (1-2), 188–97. [DOI] [PubMed] [Google Scholar]
- 94.Torge A et al. (2017) Ciprofloxacin-loaded lipid-core nanocapsules as mucus penetrating drug delivery system intended for the treatment of bacterial infections in cystic fibrosis. Int J Pharm 527 (1-2), 92–102. [DOI] [PubMed] [Google Scholar]
- 95.Nafee N et al. (2014) Antibiotic-free nanotherapeutics: ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J Control Release 192, 131–40. [DOI] [PubMed] [Google Scholar]
- 96.De Leo V et al. (2018) Preparation of drug-loaded small unilamellar liposomes and evaluation of their potential for the treatment of chronic respiratory diseases. Int J Pharm 545 (1-2), 378–388. [DOI] [PubMed] [Google Scholar]
- 97.Patil HP et al. (2018) Fate of PEGylated antibody fragments following delivery to the lungs: Influence of delivery site, PEG size and lung inflammation. J Control Release 272, 62–71. [DOI] [PubMed] [Google Scholar]
- 98.Huang X et al. (2017) Protein nanocages that penetrate airway mucus and tumor tissue. Proc Natl Acad Sci U S A 114 (32), E6595–E6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang X et al. (2019) Hypoxia-tropic Protein Nanocages for Modulation of Tumor- and Chemotherapy-Associated Hypoxia. ACS Nano 13 (1), 236–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rafeeq MM and Murad HAS (2017) Cystic fibrosis: current therapeutic targets and future approaches. J Transl Med 15 (1), 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suk JS et al. (2014) Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J Control Release 178, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Osman G et al. (2018) PEGylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. J Control Release 285, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duncan GA et al. (2018) An Adeno-Associated Viral Vector Capable of Penetrating the Mucus Barrier to Inhaled Gene Therapy. Mol Ther Methods Clin Dev 9, 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hida K et al. (2011) Common gene therapy viral vectors do not efficiently penetrate sputum from cystic fibrosis patients. PLoS One 6 (5), e19919. [DOI] [PMC free article] [PubMed] [Google Scholar]