Fig. 2 |. Impact of tumour autonomous autophagy on immunity.
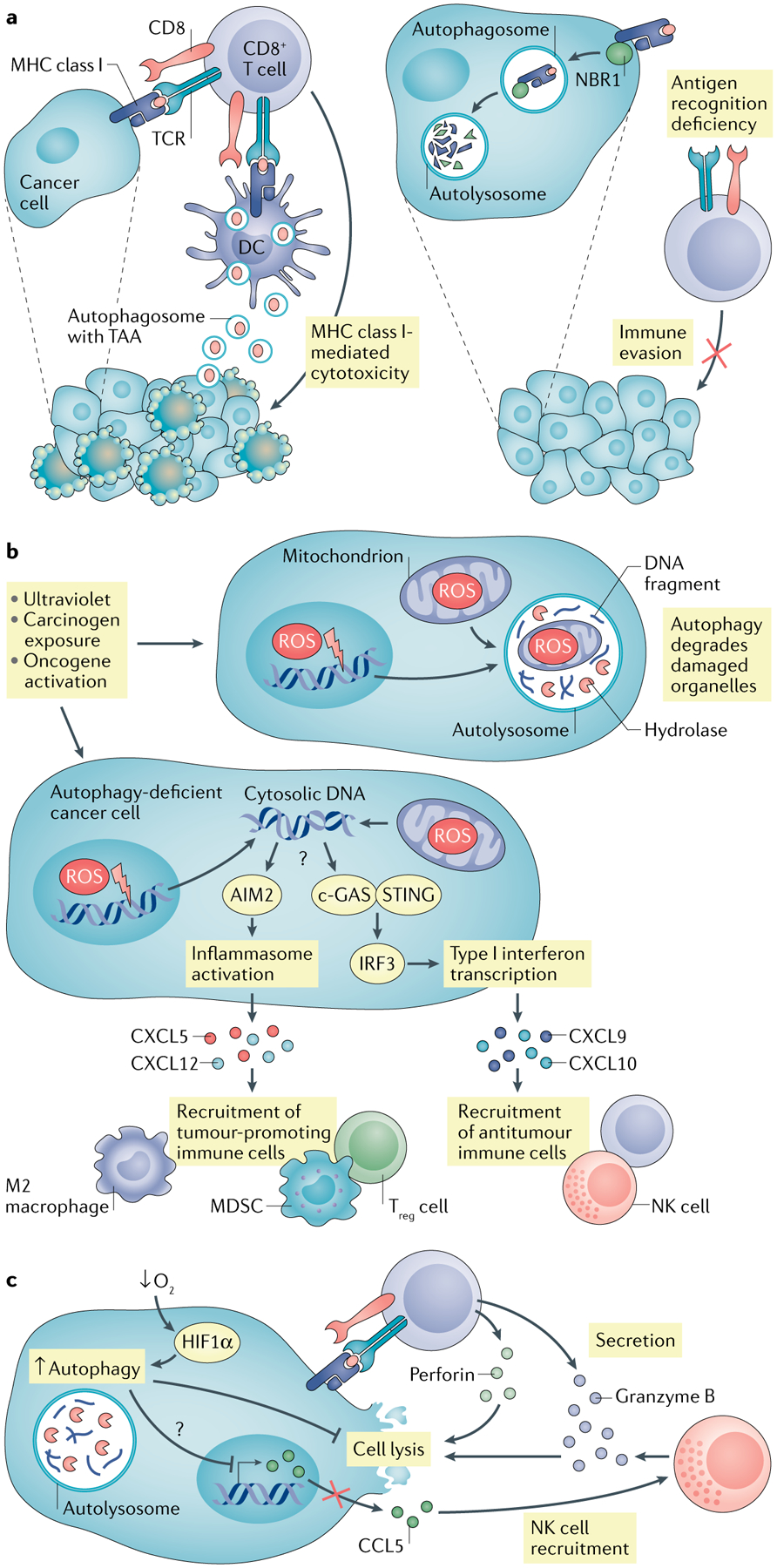
a | Autophagy in dead tumour cells promotes dendritic cell (DC)-mediated cross-presentation via increasing production of autophagosome with tumour antigen. In addition, neighbour of BRCA1 gene 1 protein (NBR1) mediates major histocompatibility complex (MHC) class I degradation via the autophagy pathway, resulting in reduced MHC class I surface expression in pancreatic cancer cells and abolished cytotoxic T lymphocyte (CTL) recognition. b | Autophagy blockade or deficiency results in accumulated reactive oxygen species (ROS) in tumour cells and subsequent mitochondria and genome damage, thereby releasing DNA into cytoplasm. Absent in melanoma 2 (AIM2) senses cytosolic DNA, activates inflammasome signalling and promotes tumorigenesis via recruiting immunosuppressive immune cells. c-GAS–stimulator of interferon genes (STING) can also sense cytosolic DNA, stimulate type I interferon responses and enhance antitumour immunity via recruiting CD8+ T and natural killer (NK) cells. c | Hypoxia induces autophagy in tumour cells through hypoxia-induced factor 1α (HIF1α). Elevated autophagy mediates degradation of granzyme B in tumour cells, which is secreted by activated CD8+ T cells and NK cells, and blocks CTL-mediated and NK cell-mediated tumour killing. Meanwhile, autophagy inhibits chemokine CCL5 expression, which recruits the NK cell migration to the tumour microenvironment (TME). MDSC, myeloid-derived suppressor cell; TAA, tumour-associated antigen; Treg cell, regulatory T cell.
