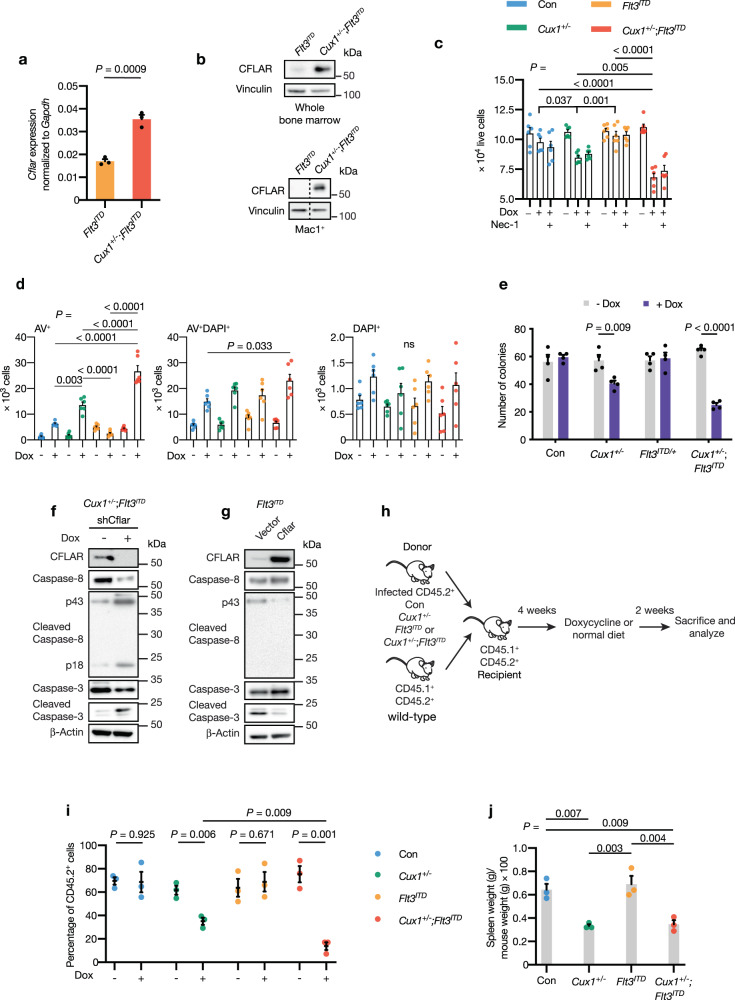
Fig. 6. CFLAR is a genetic vulnerability and restrains apoptosis in CUX1-deficient AML.
a Cflar transcript expression by qRT-PCR in Cux1+/−;Flt3ITD and Flt3ITD Mac1+ cells. Expression was normalized to Gapdh. Biological and technical triplicates were analyzed. b Immunoblot showing increased CFLAR expression in whole bone marrow (top) and Mac1+ myeloid cells (bottom) from Flt3ITD and Cux1+/−;Flt3ITD mice. Vinculin was used as a loading control. The dashed line indicates splicing of non-adjacent lanes from the same blot. The experiment was performed twice. c Growth assays using 2 × 104 c-Kit+ cells from two mice of the indicated genotypes plated in triplicate. Doxycycline (0.1 µg/ml, Dox) and/or Nec-1 (20 µM) was added and the number of live cells per well was determined by Annexin-V/DAPI staining 48 h later. d Annexin-V/DAPI staining to determine numbers of early- and late-apoptotic cells in doxycycline-treated cells from c. e Quantification of colonies derived from plating 500 c-Kit+ cells transduced with doxycycline-inducible Cflar-targeting shRNA lentiviruses from indicated mice. Cells from two mice were plated in duplicate on cytokine-supplemented methylcellulose in the presence or absence of doxycycline. Colonies containing more than 50 cells were scored seven days after plating. f Immunoblot of lysates from Cux1+/−;Flt3ITD c-Kit+ cells transduced with doxycycline-inducible Cflar-targeting shRNA lentiviruses with and without 48 h of doxycycline treatment showing differences in caspase-8 and -3 activation. The immunoblot was performed once. g Immunoblot of lysates from Flt3ITD c-Kit+ cells showing effect of stable CFLAR expression on caspase-8 and -3 activation. The immunoblot was performed once. h Experimental outline of bone marrow transplant assay to assess selective impact of Cflar depletion on Cux1+/−;Flt3ITD cells compared with control (Con), Cux1+/− and Flt3ITD cells. Hematopoietic progenitor cKit+ cells from CD45.2+ mice of each genotype were infected with doxycycline-inducible Cflar-targeting shRNA lentiviruses and 105 puromycin-selected cells were transplanted with 105 wild-type CD45.1+CD45.2+ bone marrow support cells into lethally irradiated CD45.1+CD45.2+ recipient mice. After four weeks, mice were fed a doxycycline-containing diet to induce Cflar depletion or maintained on a normal diet. Donor-derived CD45.2+ bone marrow cells were quantified two weeks later by flow cytometry. i Quantification of donor-derived bone marrow CD45.2+ cells in recipient mice (n = 6, per group) transplanted with control (Con), Cux1+/−, Flt3ITD or Cux1+/−;Flt3ITD c-Kit+ cells with and without doxycycline-induced Cflar depletion. j Spleen weights normalized to body weight of mice at the termination of the transplant. All plots show mean + /± s.e.m.; ns, not significant; two-way (c) or one-way (d, j) ANOVA with Tukey’s test for multiple comparisons, Two-tailed, unpaired t-test (a, e, i).