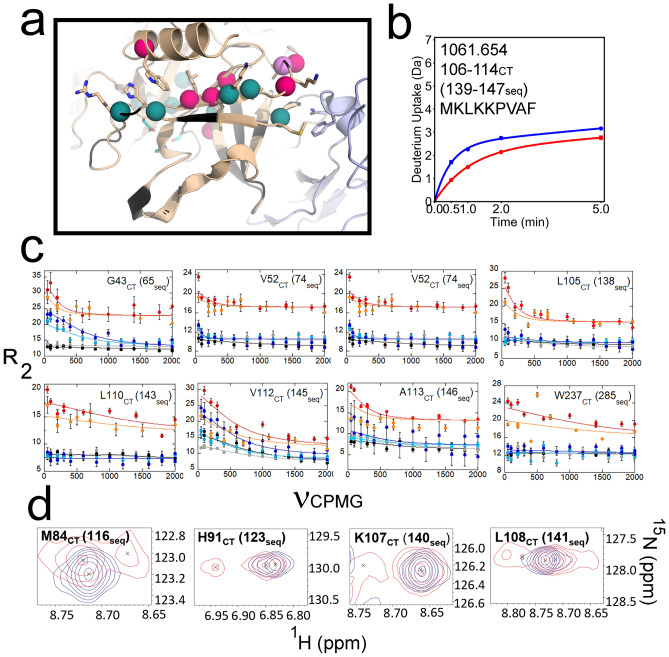
Figure 2.
(a) Structure of thrombin (wheat) bound to TM456 (light blue) [PDB ID: 1DX5]. The residues corresponding to resonances missing from the thrombin-TM456 HSQC are colored black. The residues corresponding to multiplet resonances (teal spheres), resonances experiencing increased μs-ms dynamics in thrombin-TM456 compared to apo-thrombin (pink spheres), and resonances experiencing similar μs-ms dynamics in thrombin-TM456 and apo-thrombin (violet spheres) and the catalytic triad (cyan sticks) are shown. (b) Deuterium uptake plot for the peptide spanning residues 106-114CT (139-147seq; MH + 1061.654). (c) CPMG plots for resonances in the thrombin N-terminal β-barrel (symbols as in Fig. 1). (d) Examples of thrombin-TM456 HSQC (red) doublet resonances compared to apo-thrombin (blue) resonances for residues in the N-terminal β-barrel.