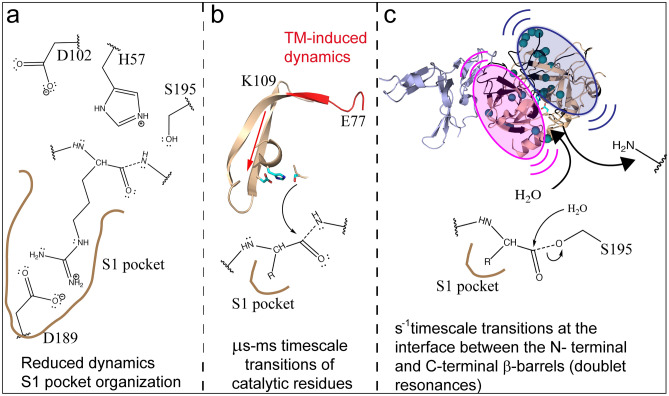
Figure 7.
A model that describes the steps of the serine protease mechanism. (a) TM decreases dynamics in the substrate binding pocket to promote protein C binding. (b) TM-induces μs-ms dynamics that may promote the optimal activity of the catalytic triad. The backbone of residues 77-84CT (108-116seq) is colored red, and the backbone of residues 56-58CT (78-80seq), 85-113CT (117–146), and 195CT (241seq) are colored wheat. The side chains of the catalytic triad are shown as cyan sticks. (c) Doublet resonances indicate TM induced dynamics on the seconds time scale of the N-terminal (pink oval) and C-terminal (blue oval) β-barrels that may be required for the release of the first product from Ser 195CT and entry of H2O into the active site for acyl enzyme hydrolysis.