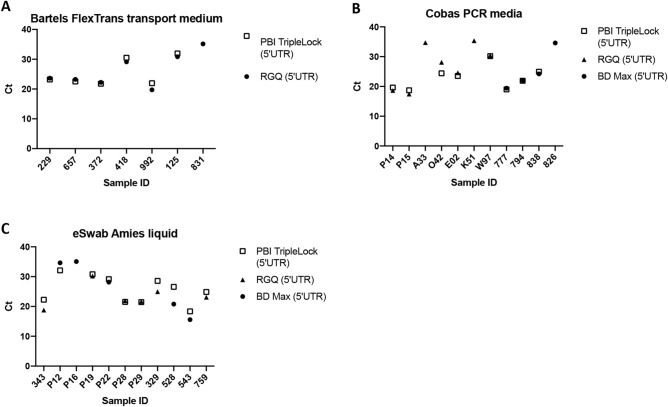
Figure 2.
Compatibility of alternate swab media with the point-of-care test. The M1 Sample Prep Cartridge Kit for RNA 2.0 was used to extract 200 ul of swabs sample from Bartels (A), Cobas (B), and eSwabs (≤ 200 µl) (C). SARS-CoV-2 was detected in 6 of 7 Bartels swabs, 10 of 11 e-Swabs, and 8 of 11 Cobas swabs. Paired t-tests comparing the Cq values of the Franklin with the Ct values of the clinical lab test detecting the same targets showed there was no significant difference between the Cq and Ct values for any swab when Ct was detected by the Franklin three9 thermocycler (pB = 0.2864, pC = 0.6566, and pE = 0.0601). pB p value of Bartels swab; pC p value of Cobas swab; pE p value of e-swab swab.