Fig. 1.
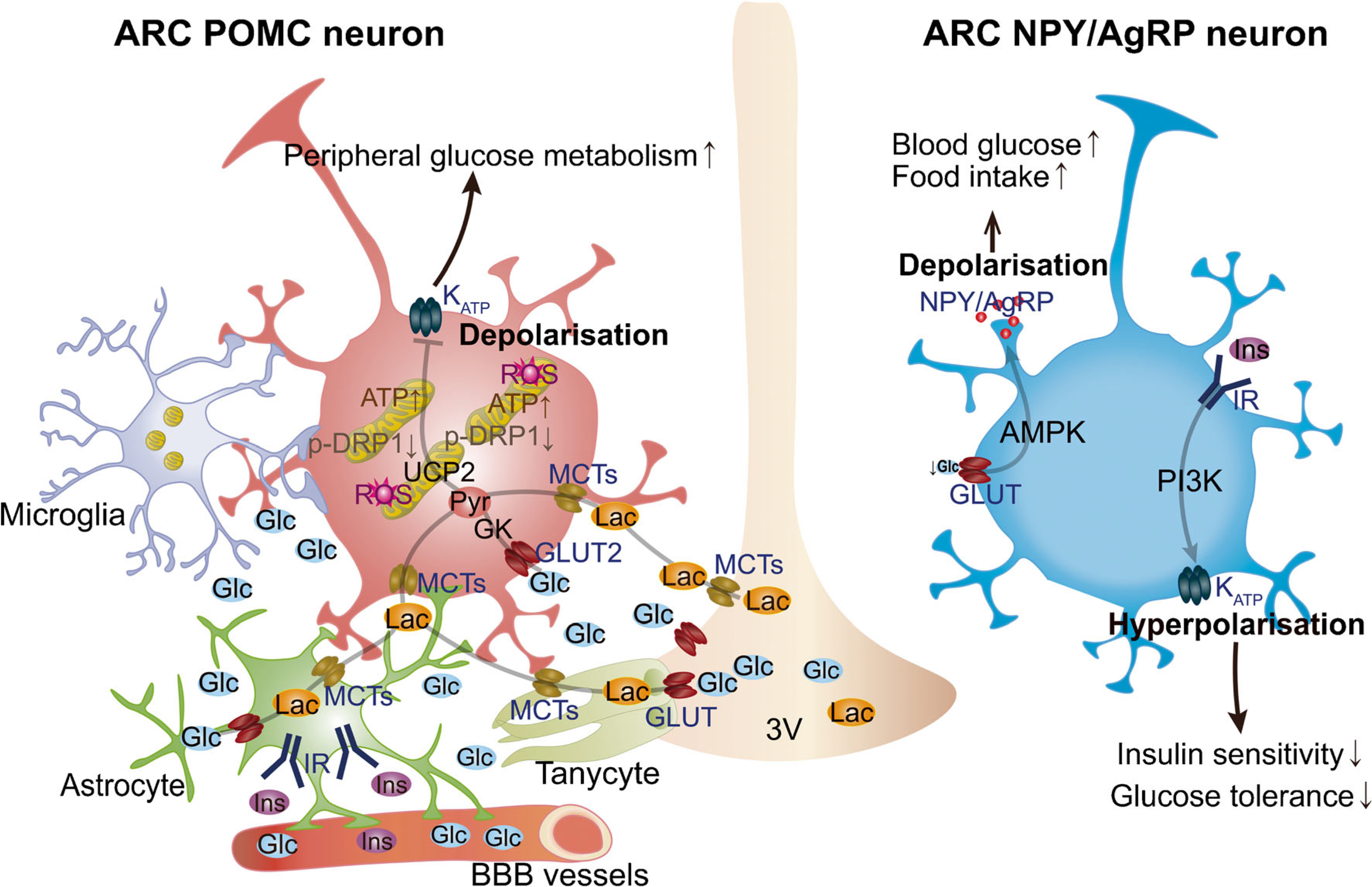
Glucose-sensing mechanisms in the hypothalamic ARC. The ability of POMC and NPY/AgRP neuronal populations in ARC to alter energy metabolism is due to their sensitivity to several circulating signals, including hormones and nutrients (e.g. glucose and lactate). Hypothalamic astrocytes provide neurons with structural support and nutrients. Astrocytic insulin signalling regulates glucose sensing via control of glucose uptake across the blood–brain barrier (BBB). Glucose is delivered to the extracellular space and transported to glial cells, such as astrocytes and tanycytes, through GLUTs and then metabolised to lactate. When released, lactate is taken up by neurons via monocarboxylate transporters (MCTs) to serve as a glycolytic substrate and metabolised into pyruvate. Hypothalamic microglia have been shown to affect POMC neuronal activation and synaptic input organisation in diet-induced obesity. Changes in glucose levels have been shown to affect POMC activity via KATP channels. Mitochondrial dynamics in hypothalamic POMC neurons has been also shown to play a pivotal role in regulating neuronal activity in response to glucose fluctuations. The activity of the ARC glucose-inhibited NPY/AgRP neurons is regulated by AMPK activity in response to decreased glucose levels, while insulin has been shown to induce hyperpolarisation of these neurons. Glc, glucose; Ins, insulin; IR, insulin receptor; Lac, lactate; PI3K, phosphoinositide 3-kinase; Pyr, pyruvate; ROS, reactive oxygen species; 3V, third ventricle.
