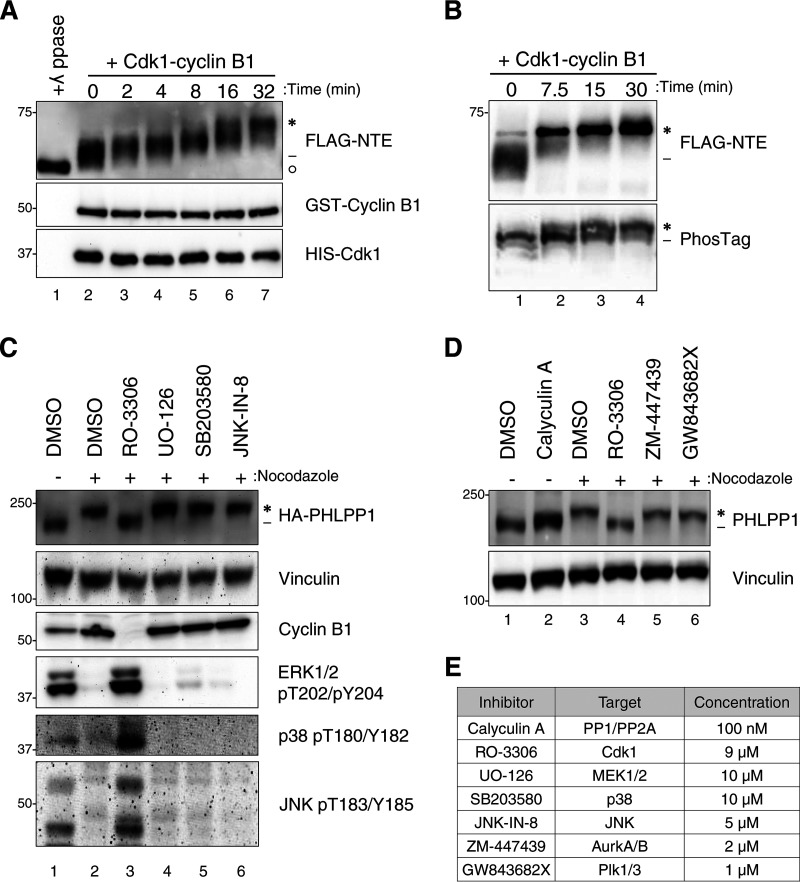
FIG 3.
Cdk1 phosphorylates PHLPP1 NTE during mitosis. (A) In vitro phosphorylation of FLAG-tagged PHLPP1 NTE with recombinant Cdk1-cyclin B1 (0.01 μg/μl). Purified NTE was incubated with either λ phosphatase (lane 1) or recombinant Cdk1-cyclin B1 (lanes 2 to 7) at 37°C for the indicated times. PHLPP1 NTE mobility shift was evaluated by Western blotting using an anti-FLAG antibody. GST-cyclin B1 and HIS-Cdk1 were probed by antibodies against cyclin B1 and Cdk1, respectively. (B) Purified NTE treated with recombinant Cdk1-cyclin B1 (0.02 μg/μl) was probed by an anti-FLAG antibody on an SDS-PAGE (top) or PhosTag (bottom) gel. (C) HeLa cells expressing HA-tagged PHLPP1 (full length) were treated with nocodazole (100 ng/ml for 16 h), followed by the following kinase inhibitors for 30 min prior to lysis: RO-3306 (Cdk1 inhibitor; 9 μM), UO-126 (MEK1/MEK2 inhibitor; 10 μM), SB203580 (p38 inhibitor; 10 μM), or JNK-IN-8 (JNK inhibitor; 5 μM). Asynchronous cells were treated with DMSO for 30 min prior to lysis. Triton-soluble lysates were subjected to Western blot analysis and probed for HA-PHLPP1. (D) HeLa cells were treated with nocodazole (100 ng/ml for 16 h), followed by various mitotic kinase inhibitors for 30 min prior to lysis as indicated: RO-3306 (Cdk1 inhibitor; 9 μM), ZM-447439 (Aurora A/B inhibitor; 2 μM), and GW843682X (Polo-like kinase 1/3 inhibitor; 1 μM). Asynchronous cells were treated with the phosphatase inhibitor calyculin A (100 nM) for 30 min prior to lysis. Triton-soluble lysates were subjected to Western blotting and probed for endogenous PHLPP1. (E) List of inhibitors used in panels C and D. Asterisks indicate PHLPP1 electrophoretic mobility shift compared to faster-mobility species (dashes). The open circle indicates PHLPP1 species after phosphatase treatment.