Figure 1: Proposed AggFluor probes can activate fluorescence differently with misfolded oligomers or insolouble aggregates via controlling their excited state rotational energy barrier.
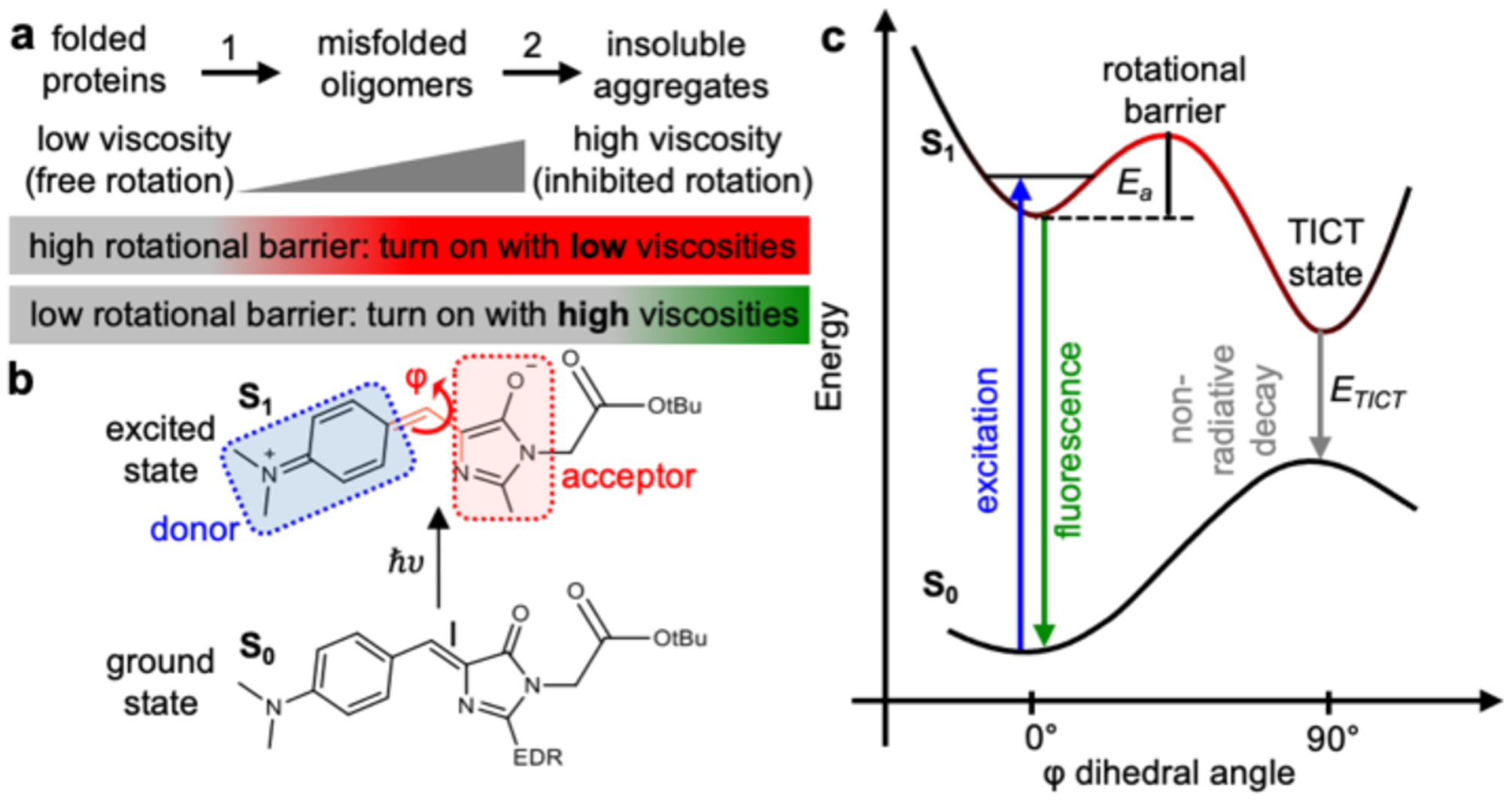
a) Diagram of how the fluorogenic AggFluor probes could visualize protein aggregation. Probes with lower barriers should activate fluorescence with both misfolded oligomers and insoluble aggregates. Whereas, probes with higher barriers should only activate fluorescence with insoluble aggregates. b) Structure of HBI at both the ground S0 and excited S1 states. At the S1 excited state, rotation occurs along the phenolate ring (P) or imidazoline ring (I) bond, wherein the prominent nonradiative decay pathway is via the rotation along the I bond as defined by the φ dihedral angle. c) The Jablonski diagram of AggFluor probes that harbor a rotational barrier between the luminescent state to the dark TICT state.
