Figure 2: AggFluor P1 harbors a rotational barrier that could explain its fluorescence activation with misfolded oligomers of lower viscosities.
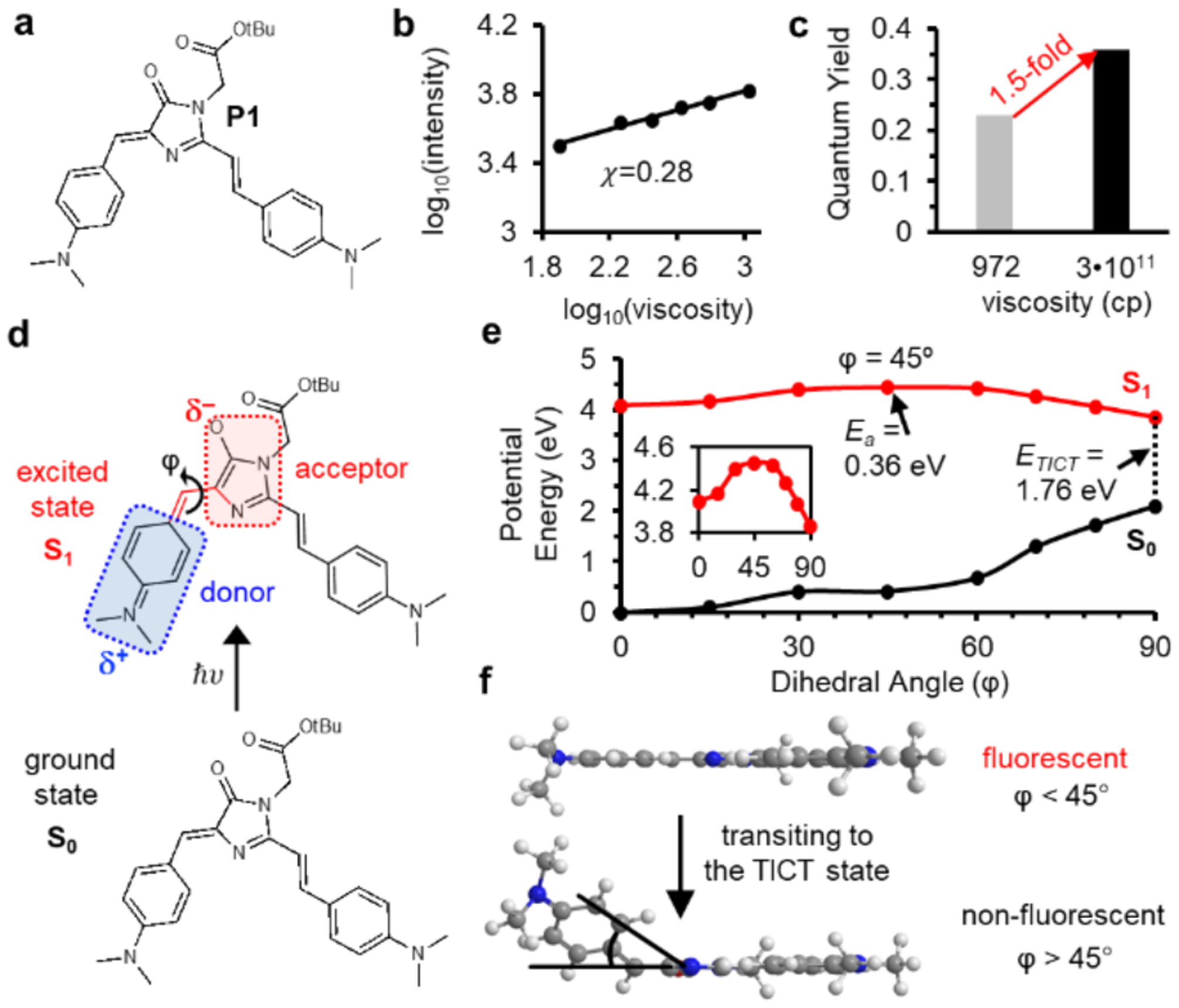
a) Structure of P1. b) Measurement of viscosity sensitivity (x) using the linear fitting of double logarithmic plots of intensity versus viscosity using ethylene glycol/glycerol mixtures, P1 shows a low viscosity sensitivity x = 0.28. c) Fluorescent intensity of P1 increase by 1.5-fold from glycerol (972 cp) to glycerol at −80°C (3•1011 cp) d). Diagram showing the donor and acceptor of P1 upon excitation. TICT state is formed via rotation between donor and acceptor along the dihedral angle φ. e) SA-CASSCF rigid scan over angle φ identifies the excited state rotational barrier at 45° with Ea = 0.36 eV. f) TICT initiates at rotational angles greater than 45°.
