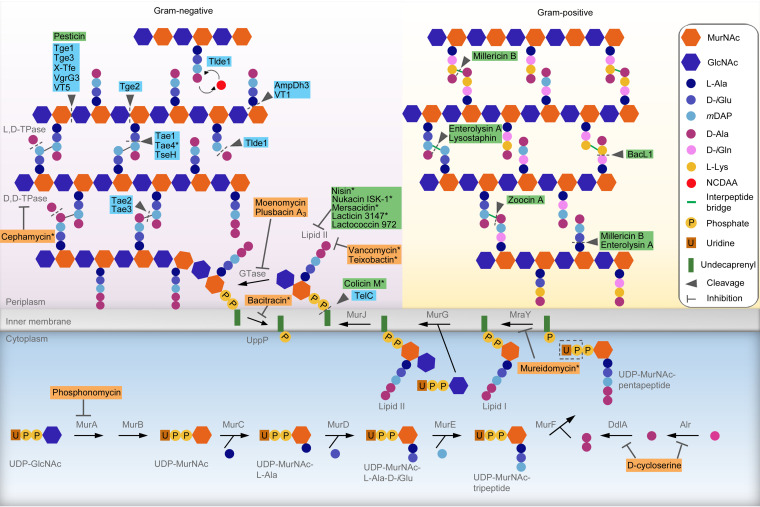
FIG 1.
Antibiotics, bacteriocins, and effectors targeting peptidoglycan synthesis and structure. Peptidoglycan precursors UDP-N-acetylglucosamine (UDP-GlcNAc) and UDP-N-acetylmuramic acid (UDP-MurNAc) are synthesized in the cytoplasm. The enzymes MurA to MurF, Alr, and DdlA are responsible for the synthesis of UDP-MurNAc-pentapeptide, which are linked to the lipid transporter undecaprenyl phosphate, forming the intermediate lipid I. Next, UDP-GlcNAc is coupled by the enzyme MurG to form lipid II, which is flipped across the cytoplasmic membrane by the flippase MurJ. The precursor lipid II is incorporated into a glycan chain by glycosyltransferases (GTases), and the undecaprenyl pyrophosphate is recycled by the enzyme UppP. Transpeptidases (TPases) are responsible for cross-linking peptide stems of the newly polymerized glycan chain to previously synthesized chains. Antibiotics (orange boxes), bacteriocins (green boxes), and contact-dependent effectors (blue boxes) targeting the peptidoglycan either by binding and inhibition or by enzymatic cleavage are indicated. Representative molecules with similar activities are indicated by asterisks, and the complete list is described in Table 1. GlcNAc, N-acetylglucosamine; MurNAc, N-acetylmuramic acid; MurA, UDP-GlcNAc enolpyruvyl transferase; MurB, UDP-MurNAc dehydrogenase; MurC, UDP-MurNAc-l-Ala ligase; MurD, UDP-MurNAc-l-Ala-d-Glu ligase; MurE, UDP-MurNAc-l-Ala-d-Glu-mesoDAP ligase; MurF, UDP-MurNAc-tripeptide-d-alanyl-d-Ala ligase; Alr, alanine racemase; DdlA, d-Ala–d-Ala ligase A; MraY, UDP-MurNAc-pentapeptide-phosphotransferase; MurG, UDP-GlcNAc-undecaprenyl-pyrophosphoryl-MurNAc-pentapeptide transferase.