FIG 3.
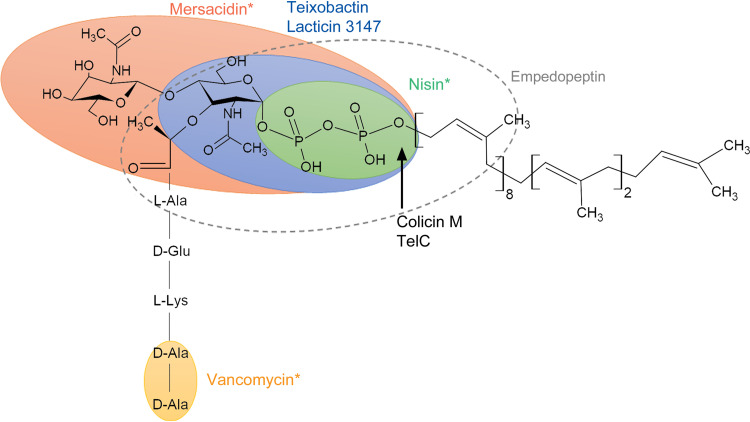
The peptidoglycan intermediate lipid II is the most common target of antimicrobials. The chemical structure of lipid II was drawn using ChemSketch software (Advanced Chemistry Development, Inc.) and is represented by GlcNAc-MurNAc-pentapeptide linked to undecaprenyl pyrophosphate. The bacteriocin mersacidin, which is representing type B lantibiotics shown in Table 1, binds to GlcNAc-MurNAc and the pyrophosphate (orange ellipse). The cyclic peptide antibiotic teixobactin and the bacteriocin lacticin 3147 (type C lantibiotic) bind to MurNAc and pyrophosphate (blue ellipse). The bacteriocin nisin, which is representing type AI lantibiotics shown in Table 1, binds to the pyrophosphate moiety of lipid II (green ellipse). The cyclic lipodepsipeptide antibiotic empedopeptin binds to the pyrophosphate moiety, MurNAc, and a portion of the peptide stem (dashed ellipse). The binding site of glycopeptide antibiotics, which are represented by vancomycin, is shown by the yellow ellipse. The arrow indicates the cleavage site of the bacteriocin colicin M and the T7SS effector TelC.