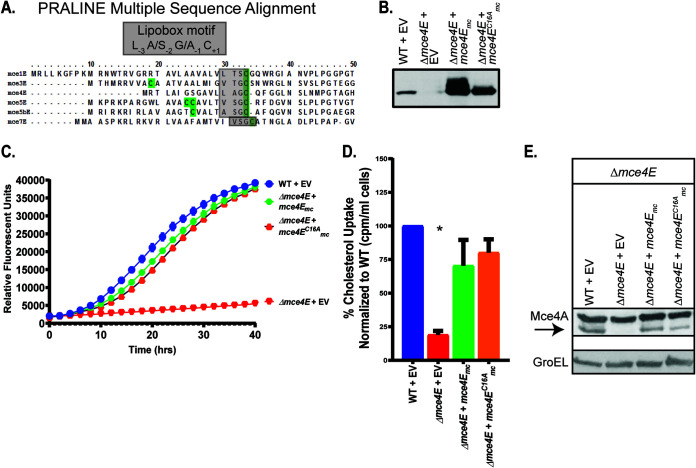
FIG 4.
The lipobox cysteine of Mce4E is not necessary for Mce4 transporter function. (A) PRALINE multiple sequence alignment of Mycobacterium smegmatis MceE proteins, with lipobox cysteines highlighted in green (63). (B) Immunoblot confirming expression by mce4E complementation plasmids. (C) Growth of 104 CFU of M. smegmatis strains on cholesterol was measured using resazurin reduction as a fluorescent readout of metabolic activity. (D) Radioactive cholesterol uptake was measured using a scintillation counter. Measurements of cholesterol uptake were normalized as a percentage of the value of WT M. smegmatis, which was set to 100%. Error bars represent standard deviations. *, P < 0.001, compared to WT results. Results are representative of at least three independent experiments. (E) Mce4A protein levels were assessed in WT, mutant, and complemented M. smegmatis strains via immunoblotting. The arrow indicates the Mce4A band, to distinguish it from the higher species cross-reacting band. GroEL levels were used as a loading control across strains. Mutant strains contain empty vectors (EV) as indicated.