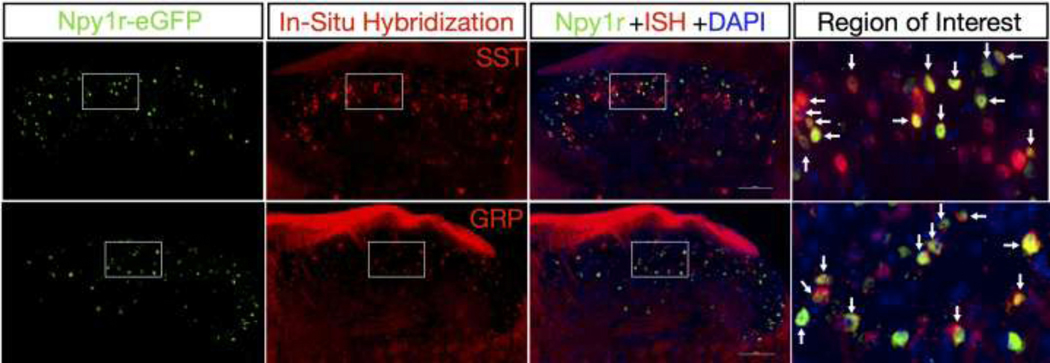
Figure 3: Npy1reGFP Interneurons Colocalize with Grp and Sst mRNA.
New data showing double staining of Npy1reGFP interneurons from BAC transgenic mice with Sst or Grp mRNA by in situ hybridization. Right panels show magnified images from their respective insets. Arrows indicate instances of colabeling. Scale bars: 100 μm. Brief description of experimental methods: 8-week-old male Npy1reGFP mice (MMRRC, 010554-UCD) were transcardially perfused with ice cold 1x PBS followed by 10% buffered formalin and spinal cords were extracted, post-fixed in 10% formalin overnight at 4°C, and then stored in 30% sucrose at 4°C. 20μm thick L3-L4 floating spinal cord sections were obtained on a vibrating microtome and mounted on Superfrost Plus Microscope slides. Slides underwent pretreatment for in situ hybridization (ISH) consisting of 10min Xylene bath, 4min 100% ethanol bath, and 2min RNAscope® H2O2 treatment. Slides were washed in deionized H2O and allowed to dry overnight. The following day the ISH protocol for RNAscope Fluorescent v2 Assay (Advanced Cell Diagnostics) was followed for hybridization to marker probes (RNAscope® Probe- Mm-Grp-C2, 317861-C2; RNAscope® Probe- Mm-Sst-C2, 404631-C2). Upon completion of ISH, slides were blocked for 1h with 3% normal goat serum and incubated overnight at 4°C with chicken anti-GFP primary antibody (Abcam, 1:1,000, ab13970) to restore the GFP fluorescence (which had diminished during fixation). The following day slides were incubated in goat anti-chicken Alexa Fluor 488 secondary antibody (Invitrogen, 1:1,000, A-11039) for 1h, and coverslipped with VECTASHIELD HardSet Antifade Mounting Medium with DAPI. All images were captured on a Nikon Eclipse Ti2 microscope using a 40x objective and analyzed using NIS- Elements Advanced Research software v5.02.