Abstract
Nintedanib is a multi‐target receptor tyrosine kinase inhibitor that reduces the decline in forced vital capacity (FVC) and prevents acute exacerbations in idiopathic pulmonary fibrosis (IPF), which is a risk factor for lung cancer. However, it remains unclear whether nintedanib is an effective treatment for lung cancer in patients with IPF. Here, we describe an 82‐year‐old man with non‐small cell lung carcinoma complicated by IPF who was treated with nintedanib. High‐resolution computed tomography (HRCT) showed a subpleural basal‐predominant reticular shadow and traction bronchiectasis with a honeycomb pattern. His FVC decreased over time, and his 6‐min walk test showed oxygen desaturation. Furthermore, an enlarged nodular lesion was detected after 6 months of referral. Biopsy confirmed non‐small cell carcinoma. Because of the risk of acute exacerbation of IPF by chemotherapy, supportive care was selected. Nintedanib was started as treatment for the IPF. Nine months later, HRCT revealed partial remission without exacerbation of IPF. This case indicates the possibility of nintedanib monotherapy in suppressing lung cancer complicated by IPF. Patients with lung cancer complicated by IPF in whom treatment is effective remain unknown. Additional research is needed to identify effective therapy for lung cancer with IPF.
Keywords: CT‐guided biopsy, idiopathic pulmonary fibrosis, lung cancer, nintedanib, non‐small cell carcinoma
We present a case of an elderly patient with non‐small cell carcinoma with underlying idiopathic pulmonary fibrosis (IPF) who was treated with nintedanib. After seven months, partial remission of the lung cancer was observed without progression of IPF. This report indicates the possibility that nintedanib monotherapy could suppress lung cancer in patients with IPF.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive lung disease and a risk factor for lung cancer development. 1 , 2 The prevalence of lung cancer in patients with IPF is 2.7%–48%. 1 Nintedanib, a tyrosine kinase inhibitor targeting fibroblast growth factor receptor, vascular endothelial growth factor (VEGF), and platelet‐derived growth factor receptor, 3 has been reported to slow down the decline of lung function and incidences of acute exacerbation. 3
However, the effects of nintedanib on lung cancer in patients with IPF remain unknown. We report a patient with non‐small cell lung cancer complicated by IPF who responded well to nintedanib.
CASE REPORT
An 82‐year‐old man with a 6‐year history of type 2 diabetes on oral medication was referred to our hospital in March 2018 following a routine chest X‐ray because of suspected interstitial lung disease. He was an ex‐smoker (1.5 pack/day from 20–55 years of age) with no history of asbestos exposure or bird rearing and no family history of interstitial lung diseases. There was no suspicion of new medication or connective tissue disease. Laboratory investigation revealed high serum levels of Krebs von den Lungen‐6 (583 U/mL) and surfactant protein D (228 ng/mL). Proteinase3‐antineutrophil cytoplasmic antibody (PR3‐ANCA) was slightly positive. However, there was no severe proteinuria or hematuria (Table 1). In September 2018, high‐resolution computed tomography (HRCT) of the chest revealed emphysematous changes in bilateral upper lobes and presence of reticular opacities and honeycombing of the bilateral lower lobes, indicative of usual interstitial pneumonia (UIP) and clinically suggesting IPF (Figure 1(a),(e)). A small nodular lesion appeared adjacent to the left lower lung lobe (Figure 1(a)). Therefore, we suspected inflammatory change; the patient was then followed‐up. In April 2019, respiratory function test revealed apparently normal predicted forced vital capacity (%FVC) and forced expiratory volume in 1 second (FEV1). However, the predicted diffusion capacity of the lung for carbon monoxide (%DLco) was reduced (60.6%), and oxygen desaturation during the 6‐minute walking test was observed (Table 1). On the other hand, the nodule in the left lower lobe increased in size from 11.94 × 11.28 × 8.87 mm3 to 30.90 × 26.04 × 21.38 mm3 (Figure 1(a),(b)). 18F‐labeled fluorodeoxyglucose (18F‐FDG)‐position emission tomography (PET)/CT revealed the largest lung tumor to have a high uptake value (SUV) of 14.6. The left lung nodules and mediastinal lymph node also had high uptake (Figure 1(c),(g),(k)). We suspected primary lung cancer or granuloma associated with granulomatosis with polyangiitis (Figure 1(a),(b),(e),(f),(i),(j)). The transbronchial lung biopsy (TBLB) from the left lower lobe tumor showed no definitive results. CT‐guided biopsy was repeated from the left lower lobe; although crushed, a diagnosis of non‐small cell carcinoma (NSCLC), favoring squamous cell carcinoma was made (Figure 2(a),(b)). We, therefore, diagnosed primary lung cancer with pleural dissemination and metastasis to the mediastinal lymph node. We suggested the anticancer agent treatment as one of the choices; however, the patient and his family declined. Furthermore, because of the patient's advanced age and the high risk of exacerbation of IPF by chemotherapy, supportive care was chosen instead. Therefore, in July 2019, to reduce lung function decline and decrease the risk of acute exacerbation in IPF, nintedanib was initiated at a dose of 100 mg twice daily. From February 2020, the dose of nintedanib was increased to 150 mg twice daily. In January 2020, chest CT revealed regression of the primary tumor (19.87 × 19.28 × 15.43 mm3), pleural dissemination, and metastasis to the mediastinal lymph node (Figure 1d,h,l). We did not expect the antitumor effect, which was observed by chance. The nintedanib caused regression of the lung cancer, and lung shadows and lung function remained stable.
TABLE 1.
Laboratory findings
| (March 2018) | ALT 7 U/L | Anti‐RNP Ab (−) | Arterial blood gas (room air) |
| Hematology | T‐bil 0.8 mg/dL | Anti‐ARS Ab (−) | pH 7.434 |
| WBC 7500/μL | LDH 176 U/L | MPO‐ANCA (−) | PaCO2 34.6 Torr |
| Neu 46.6% | CK 56 U/L | PR3‐ANCA 5.8 U/mL | PaO2 71.5 Torr |
| Lym 40.4% | Glu 166 mg/dL | HCO3 −24.0 nmol/L | |
| Mono 6.1% | HbA1c 8.4% | Urinalysis | |
| Eos 5.6% | CRP 0.11 mg/dL | Urine protein (−) | Pulmonary function (% predicted) |
| Baso 1. % | KL‐6583 U/mL | Urine OB (±) | VC 3.49 L (115.9%) |
| RBC 4.64 × 106/μL | SP‐D 228 ng/mL | Urine glucose (−) | FVC 3.45 L (118.1%) |
| Hb 14.9 g/dL | β‐D‐glucan (−) | FEV1 2.81 L (126.0%) | |
| PLT 15.5 × 104/μL | (April 2019) | FEV1/FVC 81.44% | |
| Immunology | Tumor marker | DLCO 8.95 mL/min/mm Hg (69.6%) | |
| Blood chemistry | ANA × 80 | CEA 4.04 ng/mL | DLCO/VA2.42 mL/min/mm Hg/L (60.6%) |
| TP 7.46 g/dL | Homogeneous (+) | CYFRA 3.5 ng/mL | |
| Alb 3.74 g/dL | Speckled (+) | ProGRP 45.3 pg/mL | 6‐min walking test (room air) |
| BUN 13.8 mg/dL | Ant‐CCP Ab (−) | SpO2 94% → 84% | |
| Cre 0.79 mg/dL | Anti‐SS‐A Ab (−) | Total distance 520 m | |
| AST 15 U/L | Anti‐SS‐B Ab (−) |
Abbreviations: Ab, antibody; ANA, anti‐nuclear antibody; ARS, aminoacyl‐tRNA synthetase; CCP, cyclic citrullinated peptide; CEA, carcinoembryonic antigen; CYFRA, cytokeratin fragment; DLco, diffusing capacity for carbon monoxide; FEV1, forced expiratory volume 1; FVC, forced vital capacity; MPO‐ANCA, myeroperoxidase‐antineutrophil cytoplasmic antibody; PR3‐ANCA, proteinase3‐antineutrophil cytoplasmic antibody; ProGRP, pro‐gastrin‐releasing peptide; RNP, ribonucleoprotein; SP‐D, surfactant protein‐D; SS‐A, Sjögren's‐syndrome‐related antigen‐A; SS‐B, Sjögren's‐syndrome‐related antigen‐B; VA, alveolar volume; VC, vital capacity.
FIGURE 1.
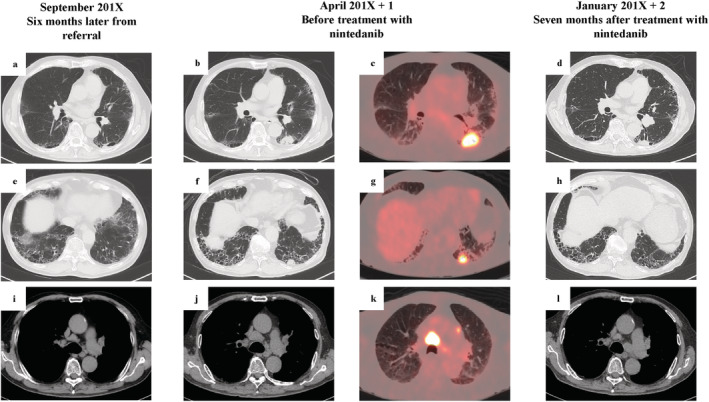
High‐resolution computed tomography (CT) images of the primary tumor (a), (b), (d), the dissemination lesion (e), (f), (h), and the mediastinal lymph node (i), (j), (l). Position emission tomography‐CT images of the primary tumor (c), the dissemination (g), and the mediastinal lymph node (k) at the time of referral (2018 Sep) (a),(e),(i), before treatment with nintedanib (2019 Apr) (b), (c), (f), (g), (j), (k)), and 7 months after treatment with nintedanib (2020 Jan) (d), (h), (l). [Correction added on 31 March 2021, after first online publication: The dates at the top of Figure 1 have been corrected from, ‘September 201X’, ‘April 201X+1’, and ‘January 201X+2’ to ‘September 2018’, ‘April 2019’, and ‘January 2020‘, respectively.]
FIGURE 2.
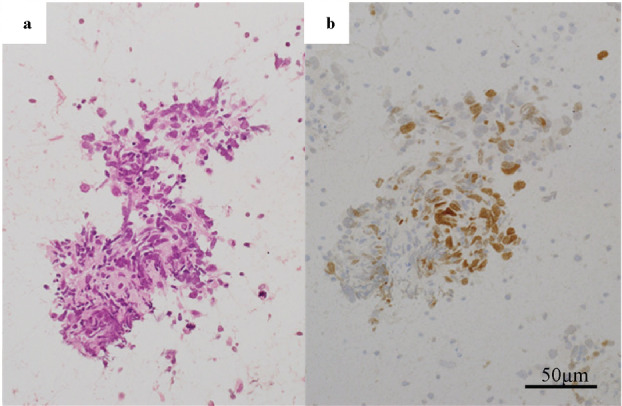
(a) Computed tomography‐guided biopsy specimen from the left lower lobe showing non‐small cell carcinoma (hematoxylin–eosin). (b) Immunohistochemistry revealed that the tumor cell was positive for p40 expression. The tumor was immunohistochemically suspected to be squamous cell carcinoma. Scale bar, 50 μm
DISCUSSION
The prognosis of patients with IPF is negatively influenced by lung cancer. In patients with IPF, nintedanib helps reduce lung function decline and decreases the frequency of acute exacerbations. 3 In several preclinical studies, chemotherapy plus nintedanib versus placebo in cases of advanced non‐small cell cancer has been reported. 4 , 5 , 6 However, these clinical studies did not evaluate the efficacy of nintedanib for the treatment of NSCLC complicated by IPF. Treatment for lung cancer complicated by IPF is often challenging because of chemotherapy‐induced exacerbation of IPF. In the present case, the main symptom of the patient was exertional dyspnea associated with IPF. There are no symptoms associated with lung cancer. Especially for elderly patients, chemotherapy is often difficult. Furthermore, because of the risk of chemotherapy, we opted for supportive care for the lung cancer and started the patient on nintedanib for IPF. Seven months later, primary tumor, pleural dissemination, and mediastinal lymph node metastasis regressed, without progression of IPF. To our knowledge, two previous reports have described nintedanib treatment in patients with NSCLC complicated by IPF. One case of squamous cell carcinoma and IPF was treated with nintedanib, 7 which inhibited tumor progression for 9 months. The second report was a case of NSCLC complicated by IPF 8 ; 1 month following nintedanib, partial remission of the primary tumor and pleural dissemination was observed. Nintedanib also promotes antitumor immunity and antitumor activity in combination with PD‐1 blockade in mice. 9
In conclusion, we encountered a patient with NSCLC and IPF who responded well to nintedanib. Further research is warranted for identifying predictive factors that may help in the selection of appropriate treatments for individual patients with lung cancer complicated by IPF.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing. No funding was received for this case report.
Kai Y, Matsuda M, Fukuoka A, et al. Remarkable response of non‐small cell lung cancer to nintedanib treatment in a patient with idiopathic pulmonary fibrosis. Thorac Cancer. 2021;12:1457–1460. 10.1111/1759-7714.13935
REFERENCES
- 1. Ballester B, Milara J, Cortijo J. Idiopathic pulmonary fibrosis and lung cancer: mechanisms and molecular targets. Int J Mol Sci. 2019;20:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J. 2015;46:1113–30. [DOI] [PubMed] [Google Scholar]
- 3. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82. [DOI] [PubMed] [Google Scholar]
- 4. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non‐small‐cell lung cancer (LUME‐lung 1): a phase 3, double‐blind, randomised controlled trial. Lancet Oncol. 2014;15:143–55. [DOI] [PubMed] [Google Scholar]
- 5. Otsubo K, Kishimoto J, Kenmotsu H, Minegishi Y, Ichihara E, Shiraki A, et al. Treatment rationale and design for J‐SONIC: a randomized study of carboplatin plus nab‐paclitaxel with or without Nintedanib for advanced non–small‐cell lung cancer with idiopathic pulmonary fibrosis. Clin Lung Cancer. 2018;19:e5–9. [DOI] [PubMed] [Google Scholar]
- 6. Forster M, Hackshaw A, De Pas T, et al. A phase I study of nintedanib combined with cisplatin/gemcitabine as first‐line therapy for advanced squamous non‐small cell lung cancer (LUME‐lung 3). Lung Cancer. 2018;120:27–33. [DOI] [PubMed] [Google Scholar]
- 7. Fukunaga K, Yokoe S, Kawashima S, Uchida Y, Nakagawa H, Nakano Y. Nintedanib prevented fibrosis progression and lung cancer growth in idiopathic pulmonary fibrosis. Respirol Case Rep. 2018;6:e00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiratori T, Tanaka H, Tabe C, Tsuchiya J, Ishioka Y, Itoga M, et al. Effect of nintedanib on non‐small cell lung cancer in a patient with idiopathic pulmonary fibrosis: a case report and literature review. Thoracic Cancer. 2020;11:1720–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kato R, Haratani K, Hayashi H. Nintedanib promotes antitumour immunity and shows antitumour activity in combination with PD‐1 blockade in mice: potential role of cancer‐associated fibroblasts. Br J Cancer. 2021;124(5):914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]


