Figure 1 –
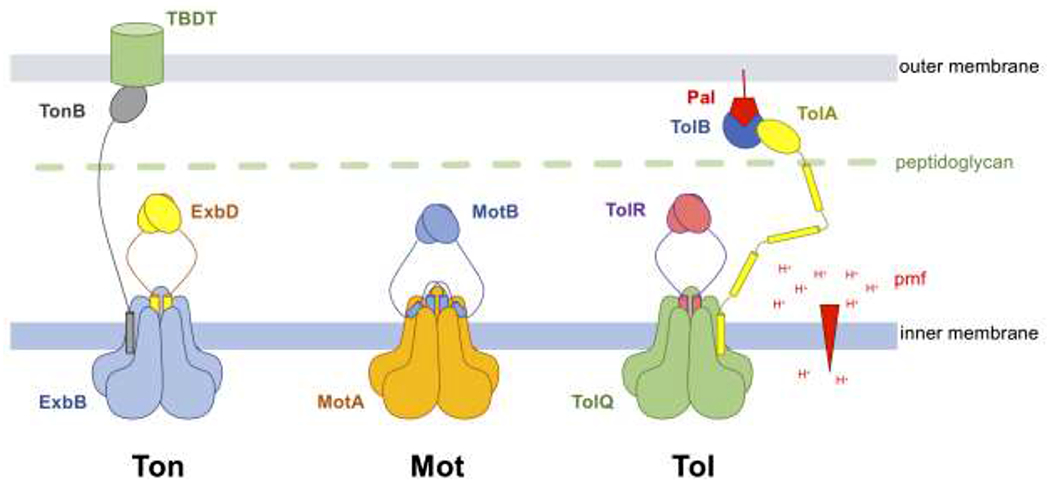
Schematic representation of the Ton, Mot and Tol complexes.
The Ton complex is composed of five ExbB (blue), two ExbD (yellow) and at least one TonB (grey). The elongated TonB subunit interacts with nutrient-loaded TBDTs at the outer membrane.
The Mot complex contains five MotA (orange) and two MotB (blue). The MotA-MotB is represented here in its plugged state. The plug domain of MotB, a short helix C-terminal of the TM domain, binds at the interface between two MotA subunits and blocks the movement of protons (the positions of the plug domains are shown according to [24]). Upon incorporation with the flagellar apparatus, the plug domains dissociate from MotA, allowing the MotB C-terminal domains to interact with the peptidoglycan layer (green).
The stoichiometry of the Tol complex is currently unknown. Because of extensive homologies with the Ton and Mot complexes, the complex is shown here to contain five TolQ (green), two TolR (purple), and at least one TolA (yellow) [21]. The elongated TolA periplasmic domain interacts with Tol-Pal (blue-red) complexes at the outer membrane.
The proton (H+) gradient across the inner membrane that forms the pmf is shown as a red arrowhead.
