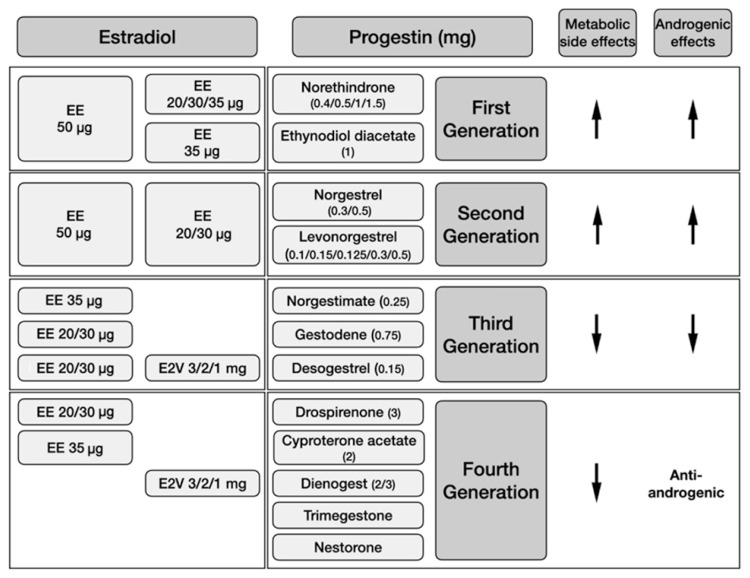
Fig. 1.
Currently available combined oral contraceptive preparations according to estradiol dosage and the type of progestin, and metabolic and androgenic side effects of the progestin component. The metabolic adverse events of combined oral contraceptives are associated with both the dose of the estradiol component and the type of progestin involved. Combinations containing lowered doses of ethinyl estradiol (EE; ≤35 μg) and more physiological forms like estradiol valerate (E2V) may be chosen in order to reduce metabolic risks. First- and second-generation progestins having androgenic and metabolic side effects are usually not favored in women with polycystic ovary syndrome. Third- and fourth-generation progestins cause fewer metabolic adverse effects, and fourth-generation progestins are also anti-androgenic. Cyproterone acetate has the greatest anti-androgen activity among all progestins.