FIGURE 1.
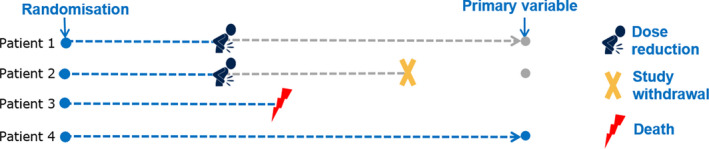
Journeys of four patients illustrating intercurrent events that occur after randomization and affect either the interpretation or the existence of the measurements associated with the primary variable. Patient 1 reduces the dose (e.g., due to an adverse event) and remains in the efficacy follow‐up. Patient 2 also reduces the dose (e.g., due to an adverse event) but withdraws at a later timepoint from further follow‐up. Patient 3 dies while on treatment
