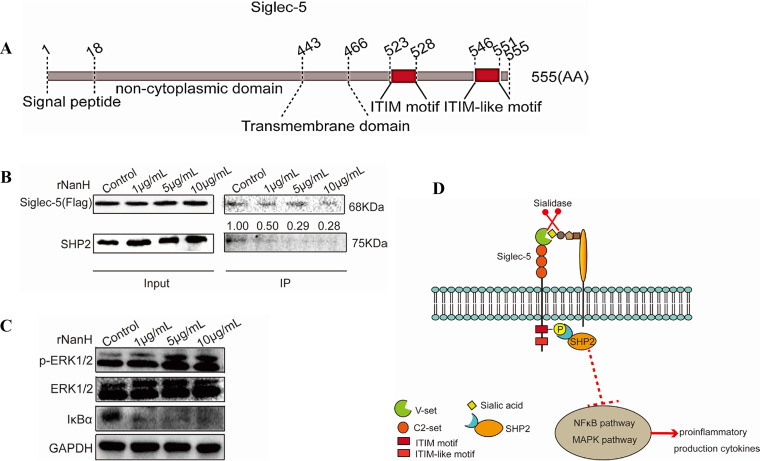
FIG 4.
Sialidase of Glaesserella parasuis abrogated the Siglec-5-mediated inhibitory signal and activated the proinflammatory signals in 3D4/21 cells. (A) Porcine Siglec-5 comprises a signal peptide (aa 1 to 18), a noncytoplasmic domain (aa 19 to 443), a transmembrane domain (aa 444 to 466), and a cytoplasmic domain (aa 447 to 555) containing an immunoreceptor tyrosine-based inhibition motif (ITIM) (aa 523 to 528) and ITIM-like motif (aa 546 to 551). (B) Siglec-5-Flag expressed by 3D4/21 cells pretreated with recombinant sialidase was coimmunoprecipitated with Flag antibody-conjugated beads and analyzed via Western blotting using anti-Flag and anti-SH2 domain-containing protein tyrosine phosphatase (SHP-2) antibodies. Reduced recruitment of SHP-2 occurred in a dose-dependent manner. (C) The 3D4/21 cells pretreated with rNanH were used for immunoblot analysis. Increased extracellular signal-regulated kinase 1/2 phosphorylation and IκBα degradation occurred in a dose-dependent manner. (D) Schematic diagram showing the activation of mitogen-activated protein kinase and nuclear factor kappa light chain enhancer of activated B cell signaling pathways via the abolishment of the Siglec-5-mediated inhibitory signal by sialidase of G. parasuis.