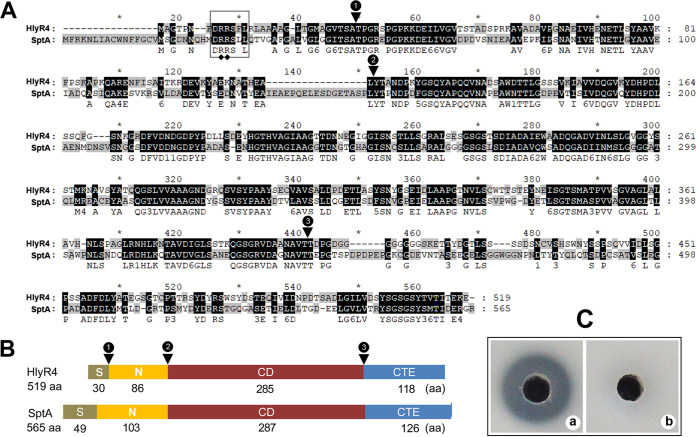
FIG 1.
Schematic representation of the primary structure of halolysin R4 (HlyR4) compared with SptA. (A) Alignment of the amino acid sequences of HlyR4 (GenBank accession no. BAA10958.1) and SptA (AAX19896.1). The Tat motif (DRRSLL) in the signal peptide is shown in a box. The representative twin-arginine structure is marked by filled diamonds. Conjunction points between the signal peptide (S), the N-terminal propeptide (N), the catalytic domain (CD), and the C-terminal extension (CTE) are marked by filled triangles. (B) Schematic diagram of HlyR4 and SptA showing the length of the S, N, CD, and CTE. (C) Proteolytic activity of HlyR4 was assessed by dripping 100 μl of untreated (a) and PMSF-treated (b) supernatants of Hfx. mediterranei ATCC 33500 into the hole of a skim milk agar plate. The final concentration of the PMSF in supernatants was 60 μg ml−1 (b).