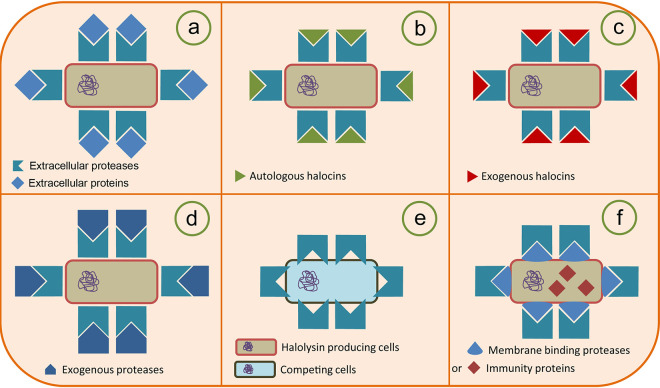
FIG 9.
Proposal for the new biological function of haloarchaeal extracellular protease by using halolysin R4 from Hfx. mediterranei (HlyR4) as an example. HlyR4, encoded by hlyR4, was initially reported as a subtilisin-like extracellular serine protease from Hfx. mediterranei. HlyR4, an extracellular protease with a primarily proteolytic activity, was secreted by Hfx. mediterranei into its surroundings to degrade proteinaceous complexes and recalcitrant substrates (e.g., protein, glycoprotein, and lipoprotein, etc.). These degradation products could then be utilized as nutrients for the secreting strain (a). Due to its proteolytic activity, the HlyR4-producing strain displayed resistance to autologous (b) or exogenous (c) halocins (proteinaceous substances). The strain was also resistant to the attack from exogenous proteases (d). The HlyR4-producing strain inhibited the growth of other haloarchaeal cells by hydrolyzing their cell surface glycoproteins (e). Strains producing HlyR4 possess some membrane-binding proteases (MBPs) on their cytomembrane. These MBPs can largely relieve the proteolytic effects from their own HlyR4, which may contribute to their survival in environments containing a certain concentration of proteases (f).