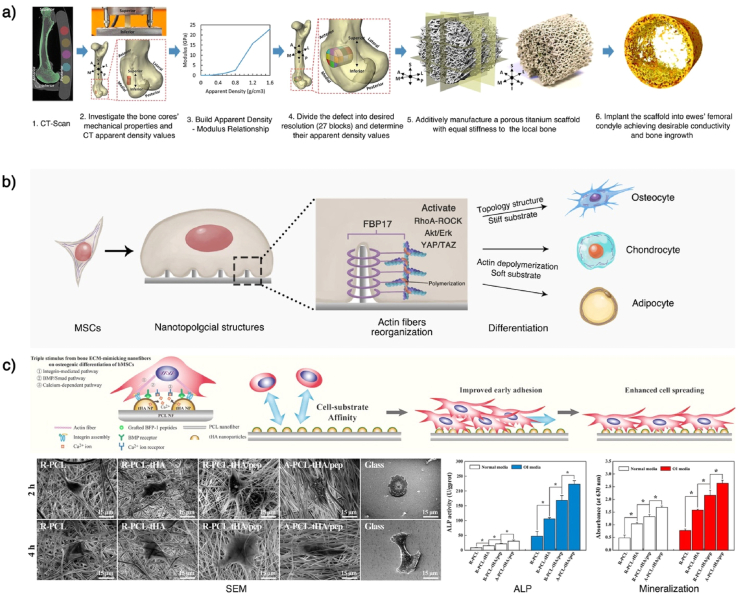
Fig. 5.
Mimic bone's mechanical and morphological structure. a) Mimicking the mechanical strength of local bone tissues. Combining a CT-scan and a mechanical test of natural bone can help establish a relationship between imageological apparent density and bone modulus, which can be used to predict the local bone mechanical strength. After predicting the local bone's mechanical strength, a stiffness-matched scaffold with desirable bone conductivity can be constructed using AM. b) Underlying regulation mechanism of scaffolds' topology structures. The surface topology can cause cell membrane curvature. The curvature can activate curvature-sensing protein FBP17, which will further induce F-actin polymerization and actin reorganization in whole-cell. This cytoskeletal system change can activate the RhoA-ROCK, Akt/Erk, and YAP/TAZ effectors of the Hippo pathway, affecting the stem cells' differentiation. The ordered topology pattern and the stiff substrate can induce osteogenic differentiation, while actin depolymerization and the soft substrate can induce chondro/adipogenic differentiation. c) Mimicking the ECM structure by nanofiber scaffolds. Topology structure nanofibers can be constructed using electrospinning and can be used to develop porous scaffolds. Gao et al. modified nanofiber scaffolds with calcium phosphate (HA), to further mimic the bone ECM and release cytokines (BMP-7) and enhance the osteogenesis effects. This scaffold was proved to promote the in vitro osteogenic differentiation of hMSCs and enhance in vivo bone formation. This Fig. was adopted from Refs. [[223], [224], [225]].