Abstract
Objective:
Dietary factors mediate racial disparities in hypertension. However, the physiological mechanisms underlying this relationship are incompletely understood. We sought to assess the association between 1-methylhistidine (1-MH), a metabolite marker of animal protein consumption, and blood pressure (BP) in a community-based cohort of black and white middle-aged adults.
Methods:
This analysis consisted of 655 participants of the Bogalusa Heart Study (25% black, 61% women, aged 34–58 years) who were not taking antihypertensive medication. Fasting serum 1-MH was measured using liquid chromatography-tandem mass spectroscopy. Animal food intakes were quantified by food-frequency questionnaires. Multivariable linear regression assessed the association between 1-MH and BP in combined and race-stratified analyses, adjusting for demographic, dietary, and cardiometabolic factors.
Results:
A significant dose–response relationship was observed for the association of red meat (P-trend <0.01) and poultry (P-trend = 0.03) intake with serum 1-MH among all individuals. Serum 1-MH, per standard deviation increase, had a significant positive association with SBP (β=3.4 ± 1.6 mmHg, P = 0.04) and DBP (β=2.0 ± 1.1 mmHg, P = 0.05) in black participants, whereas no appreciable association was observed in white participants. Among a subgroup of black participants with repeat outcome measures (median follow-up = 3.0 years), one standard deviation increase in 1-MH conferred a 3.1 and 2.2 mmHg higher annual increase in SBP (P = 0.03) and DBP (P = 0.03), respectively.
Conclusion:
Serum 1-MH associates with higher SBP and DBP in blacks, but not whites. These results suggest a utility for further assessing the role of dietary 1-MH among individuals with hypertension to help minimize racial disparities in cardiovascular health.
Keywords: 1-methylhistidine, African Americans, blood pressure, diet, hypertension, metabolomics, methylhistidines, race factors, red meat
INTRODUCTION
An estimated 103 million adults in the United States, or 46% percentage of the population, suffer from hypertension according to the 2017 American College of Cardiology/American Heart Association guidelines [1]. From 1999 to 2016, the age-adjusted prevalence of hypertension increased by nearly 10% among individuals without clinical cardiovascular disease (CVD) [2]. National surveys and trends consistently demonstrate that CVD dis-proportionately affects blacks compared with whites, as the risk of hypertension and downstream stroke, myocardial infarction, and heart failure are all higher among the US black population [3]. In particular, blacks are 40% more likely to have hypertension, 30% more likely to suffer a fatal coronary heart disease event and have twice the risk of stroke compared with whites [4]. In addition to improving equitability in the social determinants of health, identifying novel biological CVD risk factors in a race-specific fashion is an important approach that may help minimize racial disparities in CVD and improve disease prevention. The application of innovative omics research to relatively young and diverse cohorts may facilitate the development of precision health strategies for blood pressure reduction, providing an optimal approach for targeted CVD prevention.
Diet is a primary modifiable risk factor for blood pressure [5,6] and is also a robust mediator of the difference in hypertension incidence between whites and blacks [7]. In addition to the established dietary factors influencing blood pressure, including sodium [8,9], potassium [10], as well as fruits and vegetables [5], meat intake may also occupy a key role in the development, progression, and management of hypertension. Specifically, epidemiological evidence suggests that red meat and poultry consumption associate with a higher SBP and DBP [11,12]. The Dietary Approaches to Stop Hypertension (DASH) and Mediterranean diet patterns, which contain smaller amounts of red meat compared with traditional western diets, have both been associated with a reduced risk for hypertension incidence and progression [5,6,13]. Likewise, a high intake of a Southern dietary pattern, rich in fried foods, organ meats, processed meats, and egg dishes, has been noted to be an important lifestyle mediator of the higher hypertension risk in blacks versus whites [7]. Despite the presence of these data, information regarding the biological mechanisms underlying the relationship of meat intake with blood pressure, and whether this association is modified by race, remains unknown.
Urinary metabolite 1-methylhistidine correlates with red meat, poultry, and fish intake [14,15], and thus serves as an important biological marker of their consumption. Additionally, urinary 1-methylhistidine predicts vegetarian status [15], and pesco-vegetarians, lacto-ovo-vegetarians, and vegans all excrete lower urine 1-methylhistidine compared with nonvegetarians [16]. Metabolite 1-methylhistidine, also known as pi-methylhistidine (Π-methylhistidine), is among a class of organic compounds referred to as histidine and derivatives and has a half-life of approximately 12 h in humans [14,17–19]. The pi-methylhistidine chemical nomenclature refers to the concept that the nitrogen atoms of the imidazole ring of 1-methylhistidine are near, or pros (Π), in relation to the side chain [20]. Humans do not endogenously generate 1-methylhistidine in the absence of dietary meat intake [18], as 1-methylhistidine derives from anserine, a dipeptide associated with neuromuscular functioning in animal skeletal muscle [15]. Although 1-methylhistidine could help explain the documented positive associations of red meat intake with CVD and all-cause mortality, there is a paucity of research linking this metabolite to CVD or blood pressure as an upstream risk factor. Furthermore, potential race differences in these associations have not been assessed.
The current study used metabolomic profiling, food frequency questionnaires, and clinical assessments to measure the association between animal food consumption and serum 1-methylhistidine; and assess the relationship of serum 1-methylhistidine with SBP and DBP, as well as their respective longitudinal changes, in a biracial (black-white) sample of middle-aged adults of the Bogalusa Heart Study. We hypothesized that serum 1-methylhistidine would associate with red meat intake, as well as with higher SBP and DBP levels.
METHODS
Study population
The Bogalusa Heart Study, consisting of participants residing in Bogalusa, Louisiana, is an epidemiological study that observes cardiovascular health across the lifespan [21]. Between 1973 and 2016, seven surveys of children aged 4–17 as well as 10 surveys of adults, who had been previously examined as children, were conducted. The current BHS cohort (2013–2016) includes 1298 participants, born between January 1959 and June 1979 who were examined twice in both childhood and adulthood. For the current analysis, individuals were examined between 2013 and 2016. There were a total 843 BHS participants who were not taking antihypertensive medication and whom had also undergone dietary, metabolite, and blood pressure measurements. After excluding 188 individuals because of missing covariable data, we selected the 655 individuals who were not taking antihypertensive medication and who had previous blood pressure, metabolomic, nutritional, as well as respective covariable data. Among the 655 participants with cross-sectional blood pressure, metabolomic, nutritional, and covariable assessments, 132 individuals had follow-up blood pressure examinations (Supplemental Figure 1, http://links.lww.com/HJH/B399).
All study participants provided written informed consent at each examination, and study protocols were approved by the Institutional Review Board of the Tulane University Health Sciences Center.
General clinical examination
Stringent protocols were used to collect clinical data on Bogalusa Heart Study participants [22]. Validated questionnaires were used to acquire demographic and lifestyle variables specifically, age, race, sex, cigarette smoking, and alcohol consumption. Blood pressure was measured in triplicate on the right arm of participants by trained study personnel using the Omron HEM 907XL digital blood pressure device after 5 min in sitting position and avoiding caffeine, cigarettes, alcohol, eating, and physical activity for 12 h prior to the clinic visit. The mean values of each of the three SBP and DBP measurements were used for analysis. Hypertension was defined as a SBP at least 130 mmHg or DBP at least 80 mmHg [23]. Fasting measures of LDL-C, HDL-C, serum triglycerides, and HbA1c were collected using standardized procedures [24,25]. Weight in kilograms was divided by height in meters squared to calculate BMI as an assessment of adiposity. Serum creatinine was quantified using the kinetic Jaffe method. Serum creatinine was used to calculate estimated glomerular filtration rate (eGFR) via the CKD-EPI equation [26]. Semiquantitative urine protein dipstick analysis was performed on collected urine samples (Siemens Healthcare Diagnostics, Newark, Delaware, USA).
Metabolomic analysis
Fasting serum samples were collected from study participants and subsequently stored at −80 °C prior to metabolite analysis. Metabolomic analysis was performed by Metabolon Inc. (Durham, North Carolina, USA) using ultrahigh performance liquid chromatography-tandem mass spectroscopy (MS) [27,28]. Metabolite peaks for 1-methylhsitidine were quantified by leveraging the area-under-the-curve, as original values represented raw data counts. A normalization procedure was then completed to account for variation because of inter-day tuning differences in the instrument. Metabolite values for 1-methylhistidine were corrected in run-day blocks by rescaling the median to one and normalizing each data point proportionately. Detection and semi-quantification of the 1-methylhistidine analyte occurred via automated comparison of ion features to a reference library of chemical standard entries that include retention time, molecular weight (m/z), preferred adducts, and in-source fragments as well as associated MS spectra. Each participant’s raw metabolite value was divided by the standard deviation of the overall sample. Thus, each unit of 1-methylhistidine reflects a one-standard deviation change in the metabolite value.
Dietary assessment
Diet was assessed using the semiquantitative 283-item United States Department of Agriculture Delta Nutrition Intervention Research Initiative (NIRI) food-frequency questionnaire [29]. The Delta NIRI food-frequency questionnaire was specifically created and validated for adult residents of the lower Mississippi delta region. Participants were asked to indicate, on average, how often they consumed each food using 10 frequency categories: never; less than once per month; one time per month; two to three times per month; one time per week; two times per week; three to four times per week; five to six times per week; once per day; and two or more times per day. These values were all converted to weekly consumption and then multiplied by the food quantity consumed to obtain total servings consumed per week. Comprehensive analysis of nutrient intake, including dietary animal protein, sodium, potassium, vitamin E, and total energy, was performed at the Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital at Harvard Medical School, Boston, Massachusetts. Relevant animal food items from the Delta NIRI food frequency questionnaire were aggregated into predefined food groups: red meat, processed meat, poultry, fish, dairy, and eggs (Supplemental Table 1, http://links.lww.com/HJH/B399).
Statistical analysis
Study population characteristics were presented as means and standard deviations for normally distributed continuous variables, median and interquartile range for nonnormally distributed variables, and frequencies and percentage for categorical variables. Normality of continuous variables was assessed via the Kolmogorov–Smirnov test. The student’s t-test and chi-square test were used to assess differences in normally distributed continuous variables and categorical variables, respectively, between blacks and whites. Differences in nonnormally distributed continuous variables were assessed via the Wilcoxon signed-rank test. Serum triglycerides underwent natural logarithmic transformation to normalize the distribution of this covariable.
The association between animal food consumption and 1-methylhistidine was assessed using multivariable linear regression, adjusting for age, sex, race, and total energy intake [15]. The associations of 1-methylhistidine with SBP and DBP were assessed using multivariable-adjusted linear regression models, adjusting for age, sex and race (whenever appropriate), education, cigarette smoking, alcohol drinking, BMI, LDL-C, HDL-C, natural logarithm of serum triglycerides, HbA1c, eGFR, urine protein, as well as dietary factors -, sodium, potassium, total energy intake, and vitamin E. Vitamin E was included as a covariable in the model because vitamin E deficiency can lead to elevations in systemic 1-methylhistidine because of increased skeletal muscle oxidation [30]. For prospective analyses, absolute changes in SBP and DBP levels were divided by the time between the two visits to obtain their annualized changes. These outcome variables were then used as dependent variables in the multivariable-adjusted linear regression model described above. We conducted an a priori specified sensitivity analysis additionally adjusting for gait speed, a surrogate measure of skeletal muscle breakdown. In addition, we also conducted a post hoc sensitivity analysis adjusting for dietary animal protein, to assess whether 1-MH predicted higher blood pressure, independent from upstream animal protein sources. For all analyses, race and sex interactions were formally assessed by adding the respective race or sex × 1-methylhistidine interaction term to the multivariable linear regression models. We presented race-specific and sex-specific statistics when interaction was indicated.
RESULTS
Table 1 presents the baseline characteristics for the 655 Bogalusa Heart Study participants according to sex and race (mean age, 48 years; 61% women; 25% black). On average, study sample participants were overweight (BMI = 30 ± 7 kg/m2), prediabetic (HbA1c = 5.7 ± 0.8%), and had evidence of mild dyslipidemia (LDL = 115 ± 34 mg/dl) with preserved kidney function (eGFR = 94 ± 15 ml/min per 1.73 m2). The average sample SBP and DBP were 121 ± 15 and 77 ± 11 mmHg, respectively. A significantly higher proportion of black women (46%) had hypertension compared with white women (28%), and sex differences were identified among whites such that men had significantly higher SBP (P < 0.01) and DBP (P < 0.01) compared with women. Men also had significantly higher serum 1-methylhistidine compared with women. No significant difference in 1-methylhistidine was identified between white and black participants. There were several race and sex differences among CVD risk factors, including BMI, triglycerides, HDL-C, eGFR, and urine protein.
TABLE 1.
Characteristics of 655 Bogalusa metabolome study participants
| Whites (491) | Blacks (164) | P-value for race difference | |||||
|---|---|---|---|---|---|---|---|
| Variable | All (655) | White males (192) | White females (299) | Black males (65) | Black females (99) | Men | Women |
| Sociodemographic and lifestyle | |||||||
| Age (years) mean (SD) | 47.6 (5.4) | 48.7 (5.1) | 47.9 (5.1) | 45.3 (6.2) | 46.3 (5.5) | <0.01 | <0.01 |
| Posthigh school education [n (%)] | 373 (57.0) | 104 (54.2) ‡ | 201 (67.2) | 19 (29.3) ‡ | 49 (49.49) | <0.01 | <0.01 |
| Cigarette smoking [n (%)] | <0.01 | 0.47 | |||||
| Never | 340 (51.9) | 96 (50.0) | 162 (54.2) | 25 (38.5) ‡ | 57 (57.6) | ||
| Former | 197 (30.1) | 63 (32.8) | 94 (31.4) | 15 (23.0) | 25 (25.2) | ||
| Current | 118 (18.0) | 33 (17.2) | 43 (14.4) | 25 (38.5) | 17 (17.2) | ||
| Alcohol drinking [n (%)] | <0.01 | <0.01 | |||||
| Never | 72 (11.0) | 8 (4.2) | 29 (9.7) | 13 (20.0) | 22 (22.2) | ||
| Former | 200 (30.5) | 64 (33.3) | 87 (29.1) | 20 (30.8) | 29 (29.3) | ||
| Current | 383 (58.5) | 120 (62.5) | 183 (61.2) | 32 (49.2) | 48 (48.5) | ||
| Nutritional | |||||||
| 1-methylhistidine [mean (SD)] | 3.1 (1.0) | 3.4 (0.9) b | 2.9 (1.0) | 3.5 (1.1) b | 2.7 (0.9) | 0.42 | 0.14 |
| Total energy intake (kcal/day), mean (SD) | 2379.7 (1029.9) | 2720.7 (1045.8) b | 2085.2 (872.3) | 2858.4 (1124.7) b | 2293.6 (1074.6) | 0.37 | 0.08 |
| Total animal protein intake (g/day), mean (SD) | 69.2 (39.0) | 84.5 (41.9) b | 60.5 (33.8) | 76.9 (40.9) b | 60.9 (37.2) | 0.21 | 0.93 |
| Red meat intake, servings/week, median (Q1, Q3) | 2.5 (1.0, 4.0) | 3.3 (2.3, 5.3) b | 1.8 (0.9, 4.0) | 2.5 (0.9, 6.0) a | 2.0 (0.6, 3.8) | 0.08 | 0.30 |
| Processed meat intake, servings/week, median (Q1, Q3) | 6.4 (3.9, 11.9) | 8.5 (4.8, 15.6) b | 5.8 (3.5, 10.0) | 8.4 (4.6, 17.1) b | 5.0 (2.8, 9.8) | 0.99 | 0.23 |
| Poultry intake, servings/week, median (Q1, Q3) | 3.4 (1.6, 6.6) | 4.4 (2.5, 7.1) b | 2.4 (1.3, 5.5) | 6.0 (2.9, 8.6) a | 4.1 (1.8, 6.6) | 0.04 | <0.01 |
| Fish intake, servings/week, median (Q1, Q3) | 3.1 (1.8, 5.3) | 3.4 (2.0, 5.7) b | 2.8 (1.5, 4.5) | 3.3 (1.8, 6.0) | 4.0 (2.3, 6.3) | 0.84 | <0.01 |
| Dairy intake, servings/week, median (Q1, Q3) | 5.3 (2.5, 10.3) | 7.5 (3.8, 13.9) | 6.1 (2.9, 10.6) | 3.2 (1.5, 6.1) | 2.9 (1.5, 6.0) | <0.01 | <0.01 |
| Egg intake, servings/week, median (Q1, Q3) | 2.0 (1.3, 4.0) | 3.0 (1.3, 6.0) b | 2.0 (1.1, 4.0) | 2.5 (1.3, 6.0) | 1.9 (0.6, 4.0) | 0.55 | 0.46 |
| Dietary vitamin E, mg/day, mean (SD) | 12.5 (6.1) | 13.7 (6.5)‡ | 11.5 (5.7) | 13.8 (6.4) | 12.4 (5.5) | 0.93 | 0.15 |
| Dietary sodium, mg/day, mean (SD) | 4403.1 (1958.8) | 4880.9 (2032.4) b | 3968.9 (1673.7) | 5216.8 (2336.4) b | 4253.7 (1997.2) | 0.27 | 0.20 |
| Dietary potassium (mg/day), mean (SD) | 3106.2 (1369.5) | 3515.3 (1452.8) b | 2815.6 (1170.1) | 3474.5 (1463.8) b | 2948.9 (1462.7) | 0.85 | 0.41 |
| Hemodynamic | |||||||
| Hypertension [n (%)] | 263 (40.2) | 96 (50.0) b | 83 (27.8) | 39 (60.0) | 45 (45.5) | 0.16 | <0.01 |
| SBP (mmHg), mean (SD) | 120.9 (15.2) | 124.7 (12.2) b | 116.2 (14.1) | 128.4 (15.9) a | 122.7 (18.7) | 0.09 | <0.01 |
| DBP (mmHg), mean (SD) | 76.8 (10.5) | 78.9 (9.1) b | 74.3 (10.0) | 80.0 (11.9) | 78.5 (11.6) | 0.48 | <0.01 |
| Metabolic | |||||||
| BMI (kg/m2), mean (SD) | 29.7 (6.6) | 29.7 (5.3)a | 28.6 (6.3) | 30.1 (8.6)a | 32.7 (7.7) | 0.73 | <0.01 |
| LDL cholesterol (mg/dl), mean (SD) | 115.0 (34.4) | 116.5 (33.4) | 117.2 (32.7) | 113.7 (30.4) | 112.8 (42.8) | 0.54 | 0.35 |
| HDL cholesterol (mg/dl), mean (SD) | 53.6 (17.0) | 43.1 (11.9) b | 59.7 (17.0) | 52.4 (16.3) | 56.5 (15.8) | <0.01 | 0.11 |
| Hemoglobin A1c (%), mean (SD) | 5.7 (0.8) | 5.7 (0.8) | 5.6 (0.8) | 5.8 (1.0) | 5.7 (0.6) | 0.28 | 0.10 |
| Serum triglycerides (mg/dl), median (Q1, Q3) | 101.0 (73.0, 144.0) | 117.0 (84.5, 162.5) | 98.0 (73.0, 144.0) | 88.0 (66.0, 117.0) | 82.0 (63.0, 115.0) | <0.01 | <0.01 |
| Renal | |||||||
| eGFR (ml/min per 1.73 m2), mean (SD) | 94.1 (14.9) | 90.3 (13.6) a | 93.0 (12.9) | 94.5 (17.8) b | 104.2 (16.9) | 0.09 | <0.01 |
| Urine protein [n (%)] | <0.01 | 0.03 | |||||
| Negative | 524 (80.0) | 163 (84.9) | 253 (84.6) | 0 (0.0) b | 0 (0.0) | ||
| Trace | 69 (10.5) | 19 (9.9) | 25 (8.4) | 37 (56.9) | 71 (71.7) | ||
| > 30–100 mg/dl | 49 (7.5) | 7 (3.7) | 17 (5.7) | 7 (10.8) | 18 (18.2) | ||
| > 100–300 mg/dl | 11 (1.7) | 2 (1.0) | 3 (1.0) | 18 (27.7) | 7 (7.1) | ||
| >300 mg/dl | 2 (0.3) | 1 (0.5) | 1 (0.3) | 3 (4.6) | 3 (3.0) | ||
eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; n, number of individuals; Q1, quartile 1; Q3, quartile 3; SD, standard deviation.
Sex difference P value 0.05 or less.
Sex difference P value 0.01 or less.
Consumption of animal foods and relevant dietary intakes of the study sample are also highlighted in Table 1. Individuals consumed, on average, at least two servings of red meat, fish, and poultry per week. Black women and black men consumed significantly higher servings of poultry per week compared with white women (P < 0.01) and white men (P = 0.04). Men had higher intakes of total calories, total animal protein, red meat, processed meat, and poultry compared with women among both white and black participants. There were no significant differences between the two race groups with respect to weekly consumption of red meat, eggs, nor total energy, total animal protein, sodium, potassium, and vitamin E intake per day.
Follow-up assessment of blood pressure was available on 132 out of the 655 participants included in the cross-sectional analyses (Supplemental Table 2, http://links.lww.com/HJH/B399), with a median follow-up time between measurements of 3.0 years. Individuals on average had an elevated SBP (123 ± 18 mmHg) at follow-up, whereas DBP was in normal physiological range (77 ± 12 mmHg). There were no significant race differences with respect to change in SBP or DBP between the two study points. Similar to baseline, black participants had significantly higher SBP (P = 0.02) and DBP (P = 0.03) and were more likely to have hypertension at follow-up, compared with white participants. Among individuals with follow-up blood pressure measurements, 6 out of 32 black participants and 11 out 100 white participants had 1-methylhistidine values at least one standard deviation above the mean (Supplemental Table 3, http://links.lww.com/HJH/B399). Regardless of race, participants with 1-methylhistidine values at least one standard deviation above the mean had higher average baseline and follow-up SBP and DBP compared with those with lower 1-methylhistidine metabolite values.
Figure 1 presents the relationships of serum 1-methylhistidine with red meat, poultry, processed meat, fish, eggs, and dairy intake per week, after adjusting for age, sex, race, and total energy intake. A significant dose–response relationship was observed for the associations of red meat and poultry with serum 1-methylhistidine, such that systemic 1-methylhistidine linearly increased per higher tertile of red meat (P-trend <0.01) and poultry consumption (P-trend = 0.03) among all study participants. Serum 1-methylhistidine did not show a significant linear trend among tertiles of processed meat, dairy, or egg consumption. Neither race nor sex modified the associations between animal foods and serum 1-methylhistidine.
FIGURE 1.
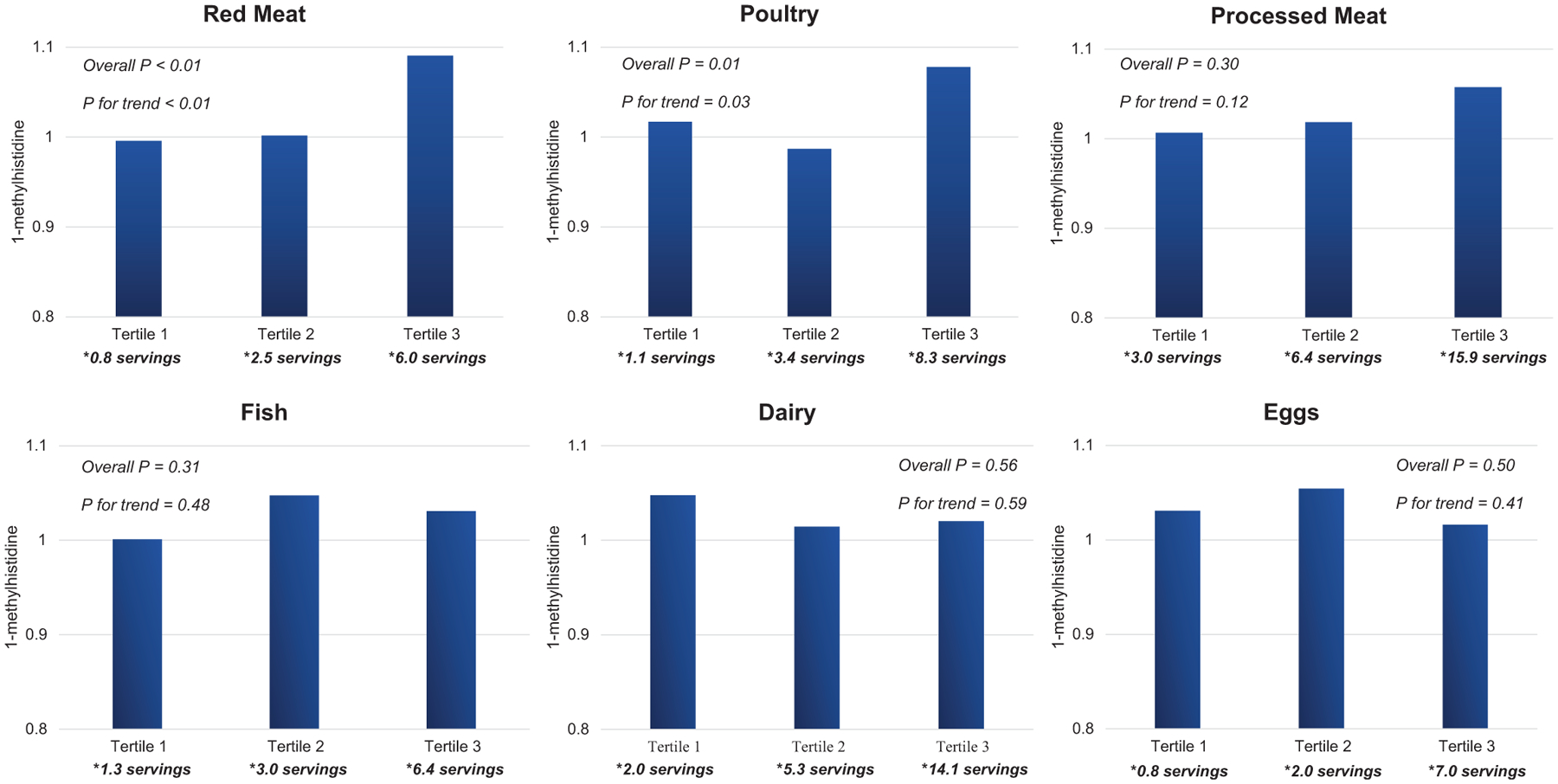
Mean serum 1-methylhistidine value according to tertile of weekly animal protein consumption, adjusted for age, sex, race, and total energy intake. *Median.
Serum 1-methylhistidine was significantly associated with both SBP and DBP in black participants, but not white participants, in cross-sectional and prospective analyses (Table 2). Overall, 1-methylhistidine had consistent parameter estimates and statistical significance with and without adjustment for dietary animal protein. For every standard deviation increase in 1-methylhistidine, we observed a 3.4mmHg higher SBP (P=0.04, P-interaction=0.04) and 2.0mmHg DBP (P=0.05, P-interaction=0.06), among black participants at baseline. Prospectively, one standard deviation increase in 1-methylhistidine significantly associated with a 3.1 and 2.2mmHg higher annualized change in SBP (P = 0.03) and DBP (P = 0.03), respectively. Parameter estimates in the cross-sectional and prospective relationships of 1-methylhistidine with SBP and DBP remained consistent in the sensitivity analyses that additionally adjusted for gait speed (Supplemental Table 4, http://links.lww.com/HJH/B399).
TABLE 2.
Cross sectional and prospective relationship of 1-methylhistidine with SBP and DBP
| Whites | Blacks | ||||
|---|---|---|---|---|---|
| Beta (SE) | P value | Beta (SE) | P value | Interaction P value | |
| Cross-sectionala | |||||
| Model without dietary animal protein c | |||||
| SBP (mmHg) | −0.6 (0.7) | 0.35 | 3.4 (1.6) | 0.04 | 0.04 |
| DBP (mmHg) | −0.4 (0.5) | 0.42 | 2.0 (1.1) | 0.05 | 0.06 |
| After adjustment for dietary animal protein d | |||||
| SBP (mmHg) | −0.7 (0.7) | 0.33 | 3.3 (1.6) | 0.04 | 0.04 |
| DBP (mmHg) | −0.4 (0.5) | 0.46 | 2.0 (1.1) | 0.05 | 0.06 |
| Prospectiveb | |||||
| Model without dietary animal protein c | |||||
| Annualized change in SBP (mmHg/year) | 0.6 (0.7) | 0.35 | 3.1 (1.2) | 0.03 | 0.69 |
| Annualized change in DBP (mmHg/year) | 0.4 (0.4) | 0.33 | 2.2 (0.9) | 0.03 | 0.61 |
| After adjustment for dietary animal protein d | |||||
| Annualizedchange in SBP (mmHg/year) | 0.6 (0.7) | 0.35 | 3.2 (1.3) | 0.03 | 0.69 |
| Annualized change in DBP (mmHg/year) | 0.4 (0.4) | 0.34 | 2.3 (1.0) | 0.04 | 0.60 |
SE, standard error.
N = 655 (491 white; 164 black).
N = 132 (100 white; 32 black).
Adjusted for age, sex, education, cigarette smoking, alcohol drinking, BMI, LDL-C, HDL-C, hemoglobin A1c, natural logarithm of serum triglycerides, eGFR, urine protein, total energy intake, and dietary-vitamin E, dietary-sodium, and dietary-potassium.
Adjusted for age, sex, education, cigarette smoking, alcohol drinking, BMI, LDL-C, HDL-C, hemoglobin A1c, natural logarithm of serum triglycerides, eGFR, urine protein, total energy intake, and dietary-animal protein, dietary-vitamin E, dietary-sodium, and dietary-potassium.
DISCUSSION
Here, we report on the first study to assess the role of serum 1-methylhistidine in blood pressure among middle-aged black and white men and women. Though dietary factors have previously been identified to be key drivers in the difference in hypertension incidence between blacks and whites [7], the mechanisms underlying such associations remain largely unexplored. In the current study, red meat and poultry consumption predicted higher serum 1-methylhistidine in a dose-response pattern among both white and black individuals. However, race modified the association between 1-methylhistidine and blood pressure. Specifically, serum 1-methylhistidine conferred an elevated hypertension risk in blacks, but not whites, predicting a respective 3.1 and 2.2 mmHg higher annualized increase in SBP and DBP for each standard deviation increase in the metabolite. This observational research suggests that targeted reduction of serum 1-methylhistidine may help to minimize racial disparities in hypertension incidence and improve CVD prevention.
Our study observed significant associations of red meat and poultry, but not fish, egg, nor dairy consumption with serum 1-methylhistidine. The manifestation of 1-methylhistidine in human body fluids is largely a product of dietary exposures, particularly red meat, chicken, and to a lesser extent fish [14,15,19,31]. These findings are consistent with previous studies involving urinary 1-methylhistidine and diet [14,15], particularly those demonstrating significantly lower levels of 1-methylhistidine excretion in vegetarians compared with nonvegetarians [16]. Metabolite 1-methylhistidine is a biological derivative of the dipeptide anserine (Fig. 2). Anserine is a dipeptide found in the skeletal muscle of mammals and is typically absent from human tissues [18]. Upon consumption, anserine undergoes cleavage to yield 1-methylhistidine and beta-alanine [19]. The human enzyme carnosinase catalyzes this process almost predominantly in plasma, and approximately 90% of dietary anserine is excreted as 1-methylhistidine via urine [31]. Carnosinase also appears to be expressed in human colonic mucosa [32], thereby possibly implicating a role for the gut microbiome in 1-methylhistidine metabolism.
FIGURE 2.
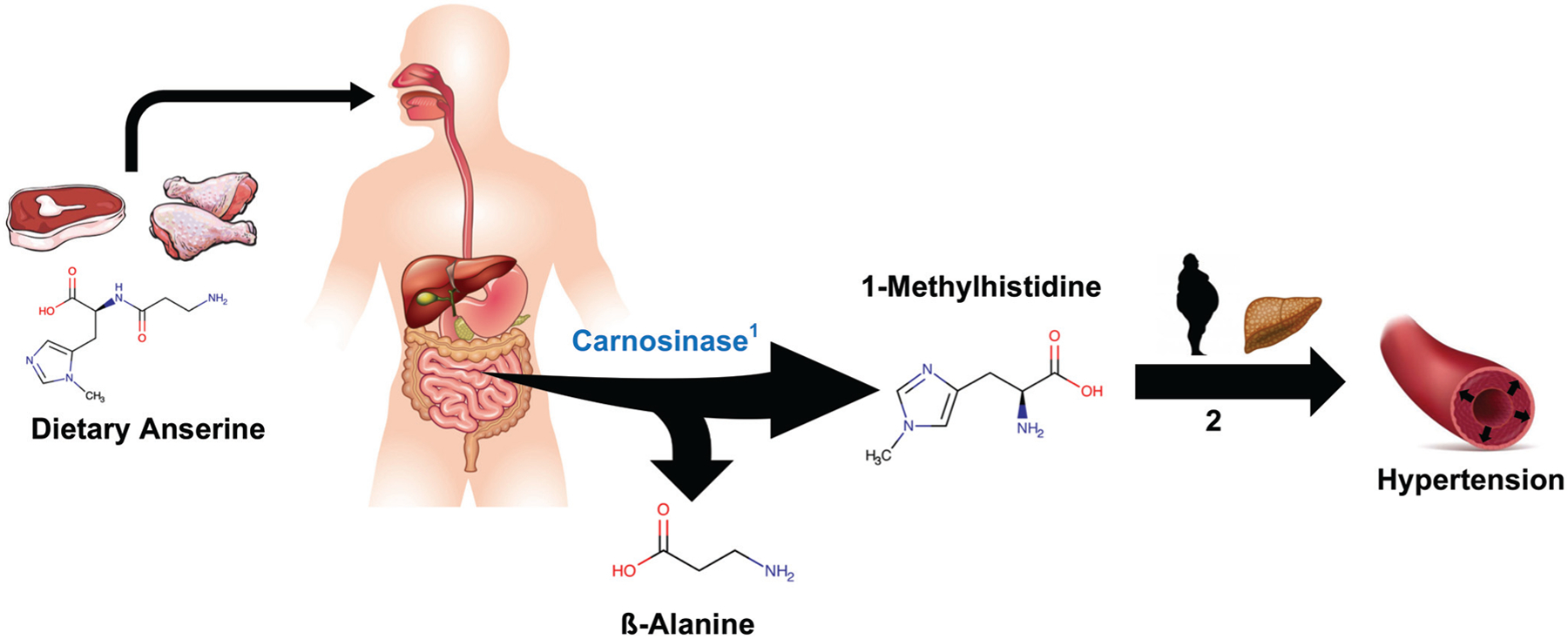
Overall scheme showing dietary precursors of 1-methylhistidine and its proposed relevance in the development of hypertension. 1. Carnosinase catalyzes conversion of dietary anserine into ß-alanine and 1-methylhistidine predominantly in systemic circulation. 2. Cumulative exposure to dietary 1-methylhistidine may increase risk for both obesity and nonalcoholic fatty liver disease, two independent risk factors for hypertension.
Findings from our cross-sectional and prospective analyses suggest that serum 1-methylhistidine may be a novel and independent risk factor for elevated blood pressure in black individuals. Furthermore, the associations between 1-methylhistidine and blood pressure were highly consistent with and without adjustment for dietary animal protein. These results suggest that for a given level of animal protein intake, serum 1-methylhistidine may vary based on metabolism, providing more support for using 1-methylhistidine as a marker of red meat and poultry intake. As mentioned earlier, carnosinase expressed both in the gut and serum, holds a key upstream function in 1-methylhistidine metabolism [19,31,32] and future studies are warranted to further identify the role of this pathway in hypertension and cardiometabolic risk.
Although 1-methylhistidine has not been implicated previously in hypertension, it has been associated with related cardiometabolic phenotypes. For example, 1-methylhistidine has previously been implicated in left ventricular diastolic dysfunction, one downstream result of chronic hypertension [33]. Likewise, evidence suggests that methylhistidine may be involved in the pathogenesis of obesity, as increased urinary excretion of 3-methylhistidine and 1-methylhistidine have been described among individuals with a higher BMI [34] and in those with nonalcoholic fatty liver disease (NAFLD) [35], respectively. Insulin resistance is hypothesized to be one mechanism linking both obesity and NAFLD to hypertension, particularly through renal sodium retention [36] and heightened stimulation of the sympathetic nervous system [37]. In the current study, we adjusted for BMI as well as HbA1c when assessing the association between 1-methylhistidine and blood pressure, suggesting that the association with elevated blood pressure are independent of these previously reported mechanisms.
The observed race differences in the relationship between 1-methylhistidine and blood pressure are unclear. We did not observe significant differences in serum 1-methylhistidine values between black and white participants, and kidney function was either nonsignificantly different (black men versus white men) or higher in blacks compared with whites (black women versus white women). Given these two observations, an association between 1-methylhistidine and blood pressure with blacks but not whites may reflect differences in 1-methylhistidine metabolism, rather than differences in dietary intake or renal excretion. However, it is still possible that differential renal excretion regulation of tubule membrane channels may affect the relationship between 1-methylhistidine and blood pressure. Future physiological and genetics research studying ancestral differences in enzymes involved with 1-methylhistidine metabolism, including carnosinase, may help further build on associations identified in the current study.
This study has several important strengths. We measured serum 1-methylhistidine in a diverse community-based cohort constituted of both black and white individuals. Through this process, we were able to identify cross-sectional associations of 1-methylhistidine with SBP and DBP, relationships that were temporally supported in a subsample of Bogalusa Heart Study participants with longitudinal blood pressure data. We believe these results will serve as a starting point for future mechanistic and multiomic studies involving diet and blood pressure.
Limitations of this study, however, should also be discussed. First, this was an observational study, and thus residual confounding is important to consider. It is plausible that 1-methylhistidine is associated with unmeasured variables, including unknown dietary variables, or CVD risk factors that may also associate with blood pressure and hypertension. Thus, future dietary randomized controlled trials are necessary prior to conclusively stating that 1-methylhistidine independently predicts blood pressure and hypertension. Likewise, metabolite values were only measured once, thus preventing longitudinal exposure assessment. To minimize residual confounding, we conducted extensive covariable adjustments in our statistical analyses. Yet, it is possible that inaccurate measurements of dietary sodium and potassium intakes, as well as unmeasured and unknown confounders, may have influenced the relationship between serum 1-methylhistidine and blood pressure.
In conclusion, the current study characterizes the relationship between dietary metabolite, 1-methylhistidine, and blood pressure in a biracial cohort of middle aged-adults in the Bogalusa Heart Study. Our analyses demonstrate that red meat and poultry consumption exhibit dose–response relationships with serum 1-methylhistidine. Furthermore, we observed more than a 3 and 2 mmHg higher SBP and DBP per year, respectively, for each standard deviation increase in serum 1-methylhistidine among blacks, whereas no appreciable association was observed among whites. These observations suggest a utility for further assessing the role of dietary 1-MH among individuals with hypertension to help minimize racial disparities in cardiovascular health.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all staff members and study personnel whom help conduct, sustain, and continue the Bogalusa Heart Study. We are especially grateful to the Bogalusa Heart Study participants.
Previous presentations: The findings from this article were presented as a poster presentation at the 2019 Hypertension Scientific Sessions Conference hosted by the American Heart Association in New Orleans, LA.
This research was supported by several grants. A.C.R. is currently funded through a fellowship training grant supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under grant number F30HL147486. T.N.K. was supported in part by the National Institutes of Health under grant numbers: P20GM109036 and R21AG051914. L.A.B. was supported in part by the National Institutes of Health under grant numbers: K12HD043451, P20GM109036, R21AG057983, R01HL121230 and R01DK091718. J.H. was supported in part by the National Institutes of Health under grant number: P20GM109036. C.M.R. was supported in part by the National Institutes of Health: K01DK107782 and R21HL143089.
Abbreviations:
- 1-MH
1-methylhistidine
- BHS
Bogalusa Heart Study
- BP
blood pressure
- CVD
cardiovascular disease
- DASH
dietary approaches to stop hypertension
- eGFR
estimated glomerular filtration rate
- HbA1c
hemoglobin A1c
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- MS
mass spectrometry
- NAFLD
nonalcoholic fatty liver disease
- NIRI
nutrition intervention research initiative
Footnotes
Conflicts of interest
J.K., whom contributed to metabolomics data collection, is employed by Metabolon Inc. Metabolon Inc. did not participate in the study design, statistical analysis, or scientific interpretation of the current research. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, et al. Potential US population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol 2018; 71:109–118.29146532 [Google Scholar]
- 2.Dorans KS, Mills KT, Liu Y, He J. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc 2018; 7:008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, et al. , American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation 2017; 136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 4.Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev 2015; 11:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. , DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344:3–10. [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997; 336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 7.Howard G, Cushman M, Moy CS, Oparil S, Muntner P, Lackland DT, et al. Association of clinical and social factors with excess hypertension risk in black compared with white US adults. JAMA 2018; 320:1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of non-pharmacologic interventions in the elderly (TONE). J Am Med Assoc 1998; 279:839–846. [DOI] [PubMed] [Google Scholar]
- 9.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP). Br Med J 2007; 334:885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whelton PK, He J, Cutler JA, Brancati FL, Appel LJ, Follmann D, Klag MJ. Effects of oral potassium on blood pressure. Meta-analysis of randomized controlled clinical trials. JAMA 2003; 277:1624–1632. [DOI] [PubMed] [Google Scholar]
- 11.Griep LMO, Seferidi P, Stamler J, Van Horn L, Chan Q, Tzoulaki I, et al. , INTERMAP Research Group. Relation of unprocessed, processed red meat and poultry consumption to blood pressure in East Asian and Western adults. J Hypertens 2016; 34:1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steffen LM, Kroenke CH, Yu X, Pereira MA, Slattery ML, Van Horn L, et al. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 2005; 82:1169–1177. [DOI] [PubMed] [Google Scholar]
- 13.Toledo E, Hu FB, Estruch R, Buil-Cosiales P, Corella D, Salas-Salvadó J, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med 2013; 11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjölin J, Hjort G, Friman G, Hambraeus L. Urinary excretion of 1-methylhistidine: a qualitative indicator of exogenous 3-methylhistidine and intake of meats from various sources. Metabolism 1987; 36:1175–1184. [DOI] [PubMed] [Google Scholar]
- 15.Myint T, Fraser GE, Lindsted KD, Knutsen SF, Hubbard RW, Bennett HW. Urinary 1-methylhistidine is a marker of meat consumption in black and in white California Seventh-day Adventists. Am J Epidemiol 2000; 152:752–755. [DOI] [PubMed] [Google Scholar]
- 16.Fraser GE, Jaceldo-Siegl K, Henning SM, Fan J, Knutsen SF, Haddad EH, et al. Biomarkers of dietary intake are correlated with corresponding measures from repeated dietary recalls and food-frequency questionnaires in the Adventist Health Study-2. J Nutr 2016; 146:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res 2018; 46:D608–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitry P, Wawro N, Rohrmann S, Giesbertz P, Daniel H, Linseisen J. Plasma concentrations of anserine, carnosine and pi-methylhistidine as biomarkers of habitual meat consumption. Eur J Clin Nutr 2019; 73:692–702. [DOI] [PubMed] [Google Scholar]
- 19.National Institute of Health [NIH]. 1-Methyl-L-histidine, compound summary. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/1-Methyl-L-histidine. (Accessed 1 May 2019).
- 20.International Union of Pure and Applied Chemistry. IUPAC Compendium of Chemical Terminology. 2009. doi: 10.1351/goldbook. [DOI]
- 21.Allhoff P, Laaser U, Heinrich J. The Bogalusa Heart Study. In Compen- dium of lipid studies. Berlin, Heidelberg: Springer; 1991;20–21. [Google Scholar]
- 22.Bogalusa Heart Study Protocol; 1995. Available at: https://biolincc.nhl-bi.nih.gov/media/studies/bhs/Adult_Exam_Protocol.pdf?link_time=2019-04-03_12:53:51.100096. (Accessed 3 April 2019).
- 23.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan SR, Frerichs RR, Webber LS, Berenson GS. Serum lipoprotein profile in children from a biracial community: the Bogalusa Heart Study. Circulation 1976; 54:309–318. [DOI] [PubMed] [Google Scholar]
- 25.Beckamn Coulter. Beckman Coulter Glucose Procedure. Available at: https://www.beckmancoulter.com/wsrportal/techdocs?docname=/cis/BAOSR6x21A/01/EN_GLU. (Accessed 4 April 2019).
- 26.Levey AS, Stevens LA. Estimating GFR Using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010; 55:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans A, DeHaven C, Barrett T. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative. Anal Chem 2009; 81:6656–6667. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Y, Yu B, Alexander D, Thomas HM, Gerardo H, Jennifer AN, Eric B. Metabolomics and incident hypertension among blacks. Hypertension 2013; 62:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker KL, Maras J, Champagne C, Connell C, Goolsby S, Weber J, et al. A regional food-frequency questionnaire for the US Mississippi Delta. Public Health Nutr 2005; 8:87–96. [PubMed] [Google Scholar]
- 30.Fink K, Williams AD, Fink RM. 1-Methylhistidine excretion by vitamin E-deficient rabbits. J Biol Chem 1959; 234:1182–1185. [PubMed] [Google Scholar]
- 31.Hiroki A, Emiko O, Hideo S, Akio M, Shohei Y. Human urinary excretion of l-histidine-related compounds after ingestion of several meats and fish muscle. Int J Biochem 1993; 25:1245–1249. [DOI] [PubMed] [Google Scholar]
- 32.Sadikali F, Darwish R, Watson WC. Carnosinase activity of human gastrointestinal mucosa. Gut 1975; 16:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Razavi AC, Bazzano LA, He J, Fernandez C, Whelton SP, Krousel-Wood M, et al. Novel findings from a metabolomics study of left ventricular diastolic function: the Bogalusa Heart Study. J Am Heart Assoc 2020; 9:e015118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elliott P, Posma JM, Chan Q, Garcia-Perez I, Wijeyesekera A, Bictash M, et al. Urinary metabolic signatures of human adiposity. Sci Transl Med 2015; 7:285.ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Troisi J, Pierri L, Landolfi A, Marciano F, Bisogno A, Belmonte F, et al. Urinary metabolomics in pediatric obesity and NAFLD identifies metabolic pathways/metabolites related to dietary habits and gut-liver axis perturbations. Nutrients 2017; 9:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soleimani M. Insulin resistance and hypertension: new insights. Kidney Int 2015; 87:497–499. [DOI] [PubMed] [Google Scholar]
- 37.Reaven GM, Hoffman BB. A role for insulin in the aetiology and course of hypertension? Lancet (London, England) 1987; 2:435–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


