Abstract
Chorea-Acanthocytosis (ChAc) is a devastating, little understood, and currently untreatable neurodegenerative disease caused by VPS13A mutations. Based on our recent demonstration that accumulation of activated Lyn tyrosine kinase is a key pathophysiological event in human ChAc cells, we took advantage of Vps13a−/− mice, which phenocopied human ChAc. Using proteomic approach, we found accumulation of active Lyn, γ-synuclein and phospho-tau proteins in Vps13a−/− basal ganglia secondary to impaired autophagy leading to neuroinflammation. Mice double knockout Vps13a−/− Lyn−/− showed normalization of red cell morphology and improvement of autophagy in basal ganglia. We then in vivo tested pharmacologic inhibitors of Lyn: dasatinib and nilotinib. Dasatinib failed to cross the mouse brain blood barrier (BBB), but the more specific Lyn kinase inhibitor nilotinib, crosses the BBB. Nilotinib ameliorates both Vps13a−/− hematological and neurological phenotypes, improving autophagy and preventing neuroinflammation. Our data support the proposal to repurpose nilotinib as new therapeutic option for ChAc patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40478-021-01181-y.
Keywords: Chorein, Lyn, Cell signaling, Basal ganglia, Neurodegeneration
Introduction
The ultra-rare neurodegenerative disease Chorea-Acanthocytosis (ChAc) with 1000–5000 cases worldwide is one of the core neuroacanthocytosis syndromes (NA) [26, 50, 70]. NA diseases manifest with a spectrum of neurological symptoms in addition to the presence of misshaped red blood cells (RBCs) with thornlike protrusions, referred to as acanthocytes [7, 26, 35, 50]. Autosomal-recessive ChAc is caused by loss-of-function mutations in the vacuolar protein sorting 13 homolog A (VPS13A) gene encoding the chorein polypeptide gene product [8, 10, 54, 68]. Only symptomatic treatment is currently available to modify the devastating disease course [71] despite a shortened life-span marked by considerable morbidity and compromised independent living. These clinical manifestations are accompanied by loss of striatal medium spiny neurons [38] and a distinctive cortical neurodegeneration [39]. Other members of the vacuolar protein sorting (Vps) family of proteins have been linked to more common neurodegenerative disorders such as Parkinson’s disease (PD) (Vps35 and Vps13c) and frontotemporal dementia (Vps4B) [34, 60].
ChAc patients often present with progressive movement disorders like chorea, parkinsonism and/or dystonia [13, 53, 70]. Therefore, ChAc should be considered as a relevant differential diagnosis of Huntington’s disease (HD) [22]. This disease severely affects independent living, and results in significant morbidity and a markedly reduced life-span [71].
Although studies in mammalian cell lines and in model organisms such as yeast suggest that chorein’s possible function as a lipid transporter at organelle contact sites, possibly mediating non-vesicular phospholipid transport [14, 32, 74], the function of chorein remains incompletely understood. We recently identified accumulation of active Lyn, a kinase of the Src family tyrosine kinases as key driver of ChAc pathophysiology (for review see [50]). We also found that Lyn inhibition by PP2 or dasatinib (1) ameliorates the distorted erythroid morphology and other altered red cell features in vitro; and (2) ameliorates the pathologically increased synaptic transmission in striatal medium spiny neurons generated from ChAc iPSCs [9, 42, 64]. The proteotoxic effect of Lyn gain-of-function reflects impaired autophagy, in agreement with VPS13A-deficient cell models.
Here, we studied Vps13a−/− mice lacking chorein. Vps13a−/− mice display biological features of human ChAc. We confirmed the accumulation of active Lyn in both RBCs and basal ganglia of Vps13a−/− mice, associated with impaired autophagy and accumulation of phospho-Tau proteins and γ-synuclein. Dasatinib treatment of Vps13a−/− mice blocked Lyn activity in RBCs but not in the basal ganglia. Mass spectrometric analysis revealed that dasatinib did not accumulate to detectable levels in basal ganglia, thus providing one potential explanation for the absence of an effect of dasatinib on neurologic phenotype of ChAc patients. We therefore tested the second generation TKI nilotinib, with higher selectivity for Lyn and able to cross the blood–brain barrier (BBB) [21]. We showed that nilotinib reached the basal ganglia, where it inhibited Lyn and improved autophagy, neuronal loss and neuroinflammation.
Materials and methods
Mouse model for ChAc (Vps13a−/− mice)
Experiments were performed on age and gender- matched WT (C57BL/6J) and Vps13a−/− mice and on 12 months-old sex-matched Vps13a−/−Lyn−/− mice. We obtained Vps13a heterozygous knock out (±) mice from the EMMA Consortium (Additional file 1: Fig. S1). Vps13a−/−Lyn−/− mice were generated in house backcrossing for at least 16 generation Vps13a−/− mice and Lyn−/− mice. The Institutional Animal Experimental Committee of University of Verona (CIRSAL) approved the experimental protocol. Whenever indicated WT and Vps13a−/− mice were daily treated with vehicle (tap water) or dasatinib or nilotinib. Dasatinib (50 mg/Kg) was administered by daily gavage to 12 months-old mice for 1 month. Nilotinib (25 mg/Kg/day) was administered by gavage to 11 months-old Vps13a−/− mice for either 6 weeks or 3 months or 6 months. This dosage was chosen based on a previous report on a mouse model for PD and AD [20, 40]. Isoflurane-anesthetized mice were randomly assigned to experimental groups and blindly analyzed. Hematologic parameters and red cell indices were evaluated on a Siemens ADVIA 2120 hematology analyzer. Hematocrit and hemoglobin were manually determined [27, 43, 44]. Acanthocyte evaluation and counting were performed on McGrawald-Giemsa-stained blood smears and by electron microscopy [42]. Brains immediately removed from euthanized mice were dissected to isolate basal ganglia (BG) and cortex tissues, which were instantly frozen in liquid nitrogen.
Statistical analysis
Statistical analysis was performed with the GraphPad Prism 8.0 program. Data were analyzed using either t-test or one-way ANOVA (Dunnet’s test) for longitudinal studies or 2-way analysis of variance (ANOVA) with Bonferroni connection for repeated measures among mice of different genotypes. p < 0.05 was considered significant.
Behaviour test
Comparisons between two groups were performed using the two-tailed unpaired Student’s t-test. Data were expressed as the mean ± SEM. Statistical significance was accepted at the 95% confidence level (p < 0.05). Spontaneous locomotor activity was evaluated using a two-way mixed-model ANOVA (strain*day) followed by the Bonferroni post hoc test. For the analysis of gait parameters, the means of the hind and front paws and individual paws were considered. The individual averages for each mouse were calculated over three good runs (straightforward and without hesitations), and the differences between groups were evaluated with a two-tailed unpaired Student’s t-test. Statistical significance was accepted at the 95% confidence level (p < 0.05).
Immunofluorescence microscopy
Quantification of NeuN positive cells and Iba1 positive cells was followed by statistical analyses applying two-tailed Unpaired t test. Data are shown as mean ± SEM. Quantification of beclin1-positive and γ-Synuclein-positive cells were followed by a statistical analysis. Statistically significant differences between the two non-parametric data sets were assessed by Mann–Whitney’s test. Statistical significance was determined at p < 0.05.
Brain spectroscopy
Comparisons between two groups were performed using the two-tailed unpaired Student’s t-test. Data were expressed as the mean ± SEM. Statistical significance was accepted at the 95% confidence level (P < 0.05).
NanoLC/MS–MS analysis
Statistical analysis was performed in MeV using a Student’s two tailed t-test. Statistical significance was determined at P < 0.05.
Results
Vps13a−/− mice recapitulate key features of patients suffering from ChAc
Survival of Vps13a−/− mice was significantly reduced compared to wild-type animals as assessed by log-rank test analysis (Additional file 1: Fig. S1.2A). Weights of both male and female Vps13a−/− mice of across all ages were lower than those of wild-type animals (Additional file 1: Fig. S1.2B).
Hematologic phenotype
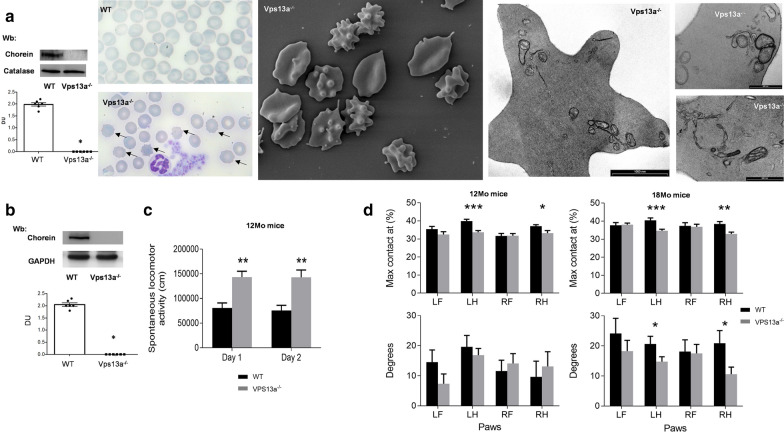
Chorein expression was undetectable in Vps13a−/− mouse RBCs (Fig. 1a). Acanthocytes were observed by multiple imaging approaches (Fig. 1a). The numbers of acanthocytes in Vps13a−/− mice were stable beyond age 2 months, considered as adult subjects from the perspective of hematologic development (Additional file 1: Fig. S1.2C). No major differences in hematologic parameters or red cell indices were observed in Vps13a−/− mice compared to wild-type animals with the exception of the Hb distribution width (HDW), useful to evaluate acanthocytes (Table 1). HDW was significantly increased in Vps13a−/− mice compared to wild-type animals (Table 1). We fractionated RBCs as a function of cell Hb content and cell volume (V/HC), revealing a dense cell fraction only in Vps13a−/− mice (Additional file 1: Fig. S1.2C, lower panel, blue circle). This fraction contains acanthocytes similarly to those observed in human ChAc [42]. As like human ChAc, osmotic fragility of Vps13a−/− mouse RBCs was higher than in wild-type RBCs (Additional file 1: Fig. S1.2D). This was associated with reduced K+ content in Vps13a−/− RBCs as compared to healthy mouse RBCs (WT mice 460 ± 12 mmol/Kg Hb vs. Vps13a−/− mice 350 ± 8.1 mmol/Kg Hb n = 6 in each group, *p < 0.05), resembling again the human disease [5].
Fig. 1.
Vps13a−/− mice exhibit hematologic and neurologic features similar to those in human ChAc. a Left panel. Western blot (Wb) analysis of chorein in red cells of WT and Vps13a−/− mice. Catalase served as protein loading control. Lower panel. Densitometric analysis (arbitrary units) of immunoblot bands like those shown; means ± SEM (n = 6; *p < 0.001 vs. WT by t-test). Right panel (from left to right). Morphologic analysis of peripheral blood from wild-type (WT) and Vps13a−/− mice. Blood smears stained with May-Grunwald-Giemsa. Cells were imaged under oil at 100 × magnification using Panfluor objective 1.30 numeric aperture on a Nikon Eclipse DS-5 M camera and processed with Digital Slide (DS-L1) Nikon. Black arrows indicate acanthocytes in Vps13a−/− mice. (see also Additional file 1: Fig. S4.2C). Electron microscopy of circulating red cells from Vps13a−/− mice. The image is representative of 10 similar imaged visual fields for each of 10 Vps13a−/− mice at the ages of 12 months. b Western blot (Wb) analysis of Chorein in isolated basal ganglia of wild-type (WT) and Vps13a−/− mice. GADPH is the loading control. Densitometric analysis (arbitrary units) of the immunoblot bands similar to those shown are presented (bottom); data are means ± SEM (n = 6; *P < 0.001 vs. WT by t-test). c Spontaneous locomotor activity in Vps13a−/− and wild-type mice in undisturbed conditions. At 12 months mice were maintained in a PhenoTyper® cage (Noldus®) and continuously monitored for two consecutive days (Day 1 and Day 2). Data represent the mean ± SEM of the total distance moved (cm) per day (**P < 0.01 vs. wild-type mice, n = 6 animals per group). d CatWalk® gait analysis of Vps13a−/− and wild-type mice. The data represent the mean ± SEM of three runs per animal and are presented per each paw, left front (LF), left hind (LH), right front (RF) and right hind (RH) (*p < 0.05, **P < 0.01, ***P < 0.001; n: 19 Vps13a−/− mice, n: 15 wild-type mice) (see also Additional file 1: Fig. S5A). At 18 months of age Vps13a−/− mice showed a deviating paw angle of both hind paws compared to controls *P < 0.05; n = 19 Vps13a−/− mice, n = 15 wild-type mice)
Table 1.
Hematological parameters and red cell indices in wild-type and Vps13a−/− mice
| Wild-type mice (n = 12) | Vps13a−/− mice (n = 12) | |
|---|---|---|
| Hct (%) | 45.3 ± 1.2 | 46.1 ± 0.8 |
| Hb (g/dl) | 14.9 ± 0.3 | 14.2 ± 0.6 |
| MCV (fl) | 52.4 ± 1.6 | 53.1 ± 1.5 |
| MCH (pg) | 16.3 ± 0.8 | 15.9 ± 0.8 |
| CHCM (g/dL) | 26.3 ± 0.2 | 26.2 ± 0.2 |
| RDW (%) | 12.5 ± 0.4 | 12.9 ± 0.7 |
| HDW (g/dL) | 2.1 ± 0.43 | 3.1 ± 0.47* |
| Retics (cell/uL) | 417 ± 65 | 382 ± 84 |
Hct haematocrit, Hb haemoglobin, MCV mean corpuscular volume, MCH mean corpuscular haemoglobin, CHCM cell hemoglobin mean content, RDW red cell distribution width, HDW hemoglobin distribution width, Retics reticulocytes *p < 0.05 compared to wild-type mice
In Vps13a−/− mouse RBCs, we found accumulation of active Lyn compared to wild-type erythrocytes (Additional file 1: Fig. S1.2E). This increase was associated with retention of double membrane remnants and vesicles, indicating an impairment of autophagy (Fig. 1a). In agreement with the morphological findings, we observed accumulation of Ulk1, Atg4, Atg5 and Rab5 polypeptides, as seen in human ChAc RBCs [42] (Additional file 1: Fig. S1.2F). Taken together these data recapitulate key hematologic features of ChAc patients.
Neurologic phenotype
Chorein was undetectable in isolated basal ganglia from saline buffer-perfused 12- and 18-months old Vps13a−/− mice (Fig. 1b). Gait and motor assessment were performed in cohorts of Vps13a−/− (n:19) and wild-type (n:15) mice at 12 and 18 months. Anxiety trait and spontaneous locomotor activity were assessed in a subgroup (n:6 for each strain) of both mouse strains (12 months-old only) applying a 5-min protocol using elevated-plus maze (EPM). No significant differences were found in the time spent in the closed and open arms (an index related to a more or a less anxiety trait) between Vps13a−/− and wild-type mice. The spontaneous locomotor activity was continuously monitored for two days by video-tracking observations of individual 12 months-old mice in PhenoTyper cages, which allowed home-cage evaluation of locomotor activity, unaffected by anxiety and/or stress of a test cage environment. The overall distance covered by the Vps13a−/− mice was significantly higher (day 1: 82.48 ± 11.68%, p < 0.01; day 2: 100.29 ± 11.68%, p < 0.01) compared to that of age-matched wild-type mice (Fig. 1c). Vps13a−/− mice and matched wild-type mice were tested for their spontaneous gait behavior (Fig. 1d). The gait performance of Vps13a−/− mice was affected during the test session at both 12 and 18 months of age. In particular, starting from 12 months, Vps13a−/− mice showed a longer duration from the start of a run until maximum contact occurs for both hind paws compared to age- and weight-matched control mice (see also Additional file 1: Fig. S2A for schematic diagram of the angle evaluation). The delay in reaching the maximum contact of paws with a glass plate was also observed at older age (Fig. 1d). Also, at 18 months, but not at 12 months (Fig. 1d), Vps13a−/− mice showed a different paw angle of both hind paws (Fig. 1d). A similar discrepancy in paw angle was reported in a transgenic rat model for Huntington’s disease [69]. “Max contact at (%)” refers to the duration, from the start of a run, until maximum contact of paws occurs. This index has been largely studied in rodent models in the context of parkinsonism [2, 3]. The basal ganglia of these aged Vps13a−/− mice exhibited accumulation of active Lyn as compared to wild-type animals (Additional file 1: Fig. S2B, C), in agreement with our previous report on active Lyn accumulation in neuronal cells derived from ChAc iPSC [64]. We note, in particular the presence in Vps13a−/− mouse basal ganglia of active Lyn stabilized in high molecular weight complexes, as earlier observed in human ChAc RBCs (Additional file 1: Fig. S2D) [42]. Collectively, our data indicate that Vps13a−/− mice recapitulate biological features and neurological phenotype like those of ChAc patients.
Vps13a−/− mice show neuronal loss and neuroinflammation
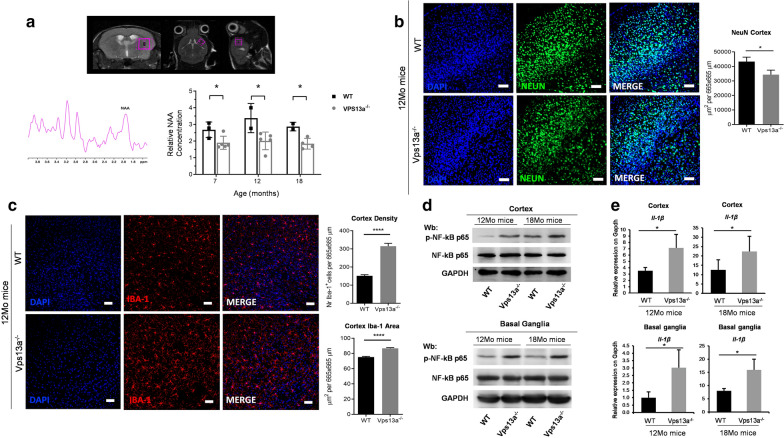
To explore neurochemical abnormalities in striatum from Vps13a−/− mice by non-invasive approaches, we used proton magnetic resonance spectroscopy H-MRS [1, 11, 31]. We found a significant reduction in N-acetylasparate (NAA) in striatum from Vps13a−/− mice compared to wild-type animals, suggesting the presence of neuronal degeneration in mice genetically lacking Chorein (Fig. 2a) [11, 51]. Histopathological studies of Vps13a−/− mice demonstrated a significant reduction in NeuN staining in cortex compared to wild-type animals, indicating a loss of neurons (Fig. 2b).
Fig. 2.
Vps13a−/− mice show neuronal loss associated with signs of neuroinflammation. a N-Acetyl Aspartate (NAA) concentration determined by 1H MRS (7 T) in the striatum of Vps13a−/− or WT control mice at different age (7, 12 and 18 months). NAA concentration was normalized using creatine as internal reference. Data are mean ± SEM (*p < 0.05 by t-test vs. WT). b Representative images of NeuN staining in cortex of WT control and Vps13a−/− mice at 12 months of age. Neurons in green, nuclei in blue. Scale bar: 50 mm (Objective 20x). Quantification of NeuN-positive cells area in cortex. Results are expressed as mean ± SEM. c Representative images of Iba-1 positive microglia cells in cortex of WT control and Vps13a−/− mice at 12 months of age (Microglia in red, nuclei in blue). Scale bar:50 mm. Quantitative analysis of microglia show significant differences in microglial density and activation in the cortex of Vps13a−/− compared to WT control mice. Results are expressed as mean ± SEM (****P < 0.0001; Unpaired t-test) d Western blot (Wb) analysis of phospho-NF-kB and total NF-kB in isolated basal ganglia of wild-type (WT) and Vps13a−/− mice at 12 and 18 months (Mo) of age. GADPH is the loading control. Densitometric analysis is shown in Additional file 1: Fig. S6. e mRNA expression of interleukine-1β (Il-1b) by qRT-PCR on cortex and basal ganglia from 12 and 18 months (Mo) old WT and Vps13a−/− mice. Experiments were performed in triplicate. Data are mean ± SD. *P < 0.05 compared with WT mice using ANOVA; internal comparisons were calculated by unpaired student t-test
It is noteworthy that we found increased microglia in the cortex from 12 months-old Vps13a−/− mice compared to wild-type animals (Fig. 2c). This was associated with increased activation of NF-kB p65 in both cortex and basal ganglia from 12 and 18 months-old Vps13a−/− mice compared to wild-type animals, consistent with neuroinflammation in mice genetically lacking Chorein (Fig. 2d, see also Additional file 1: Fig. S3). In agreement with this observation, we noted up-regulation of IL-1β mRNA expression in both cortex and basal ganglia from 12 and 18 months-old Vps13a−/− mice compared to wild-type animals (Fig. 2e).
Taken together, our data indicate neuron loss and neuro-inflammation in Vps13a−/− mice, highlighting similarities with other neurodegenerative diseases such as Parkinson disease [23].
Vps13a−/− mice show impaired autophagy involving beclin-1 pathway
To understand the possible contribution of impaired autophagy to neuronal dysfunction and neuroinflammation in Vps13a−/− mice, we evaluated expression of key autophagy flux proteins in isolated basal ganglia from saline buffer-perfused Vps13a−/− and wild-type mice. Vps13a−/− basal ganglia exhibited activation of LC3 II/I and significant accumulation of the following autophagy-related proteins: Ulk1, Atg4, Atg5, Atg9 and the lysosomal cargo protein p62 consistent with impairment of autophagy in Vps13a−/− mice (Additional file 1: Fig. S4A) as described in other neurodegenerative diseases such as PD and AD [45, 56–58]. The beclin-1 system is one of the most critical hubs of the autophagic process, playing an important role in initiation and promotion of autophagy. Severe neurodegeneration and impaired autophagy have been observed in beclin1−/− mice, further linking beclin-1-dependent autophagy with neurodegenerative diseases [45, 58]. In Vps13a−/− mice at 12 and 18 months of age, beclin-1 was significantly reduced as observed by different methods (Additional file 1: Fig. S4B, C). We also observed an accumulation of the beclin-1 complex components Vps34 and Rab5 as well as of p62, a marker of late phase of autophagy (Additional file 1: Fig. S5A). To further evaluate a possible link between chorein and beclin-1, we immunoprecipitated Beclin-1 and immunoblotted for either Chorein, Atg14L or Vps34. We found Chorein co-immunoprecipitated with Beclin-1 only in basal ganglia from wild-type mice but not from Vps13a−/− mice. In addition, we observed a reduction in Vps34 and Atg14L association with beclin-1 in Vps13a−/− mice compared to wild-type animals (Additional file 1: Fig. S5B). Noteworthy, we found up-regulation of beclin-1 mRNA levels in isolated basal ganglia from Vps13a−/− mice compared to wild-type mice, suggesting a Beclin-1 protein degradation (Additional file 1: Fig. S5C). Since Beclin-1 levels depend on Caspase 3 activity, we evaluated Caspase-3 activation by immunoblot analysis of total cleaved Caspase-3 and a fluorometric assay for Caspase-3 activity. In basal ganglia of Vps13a−/− mice, Caspase-3 activity was increased compared to wild-type animals (Additional file 1: Fig. S5D). It is of note that increased Caspase-3 activity has been also reported in brains from PD patients [4, 19]. In Vps13a−/− mice, the perturbation of autophagy resulted in accumulation of polyubiquitinated proteins in basal ganglia compared to wild-type animals, further supporting the impairment of autophagy in Vps13a−/− mice (Additional file 1: Fig. S5E).
Vps13a−/−Lyn−/− mice show reduced acanthocytes, amelioration of autophagy and decreased activity of NF-kB p65, linked to neuroinflammation
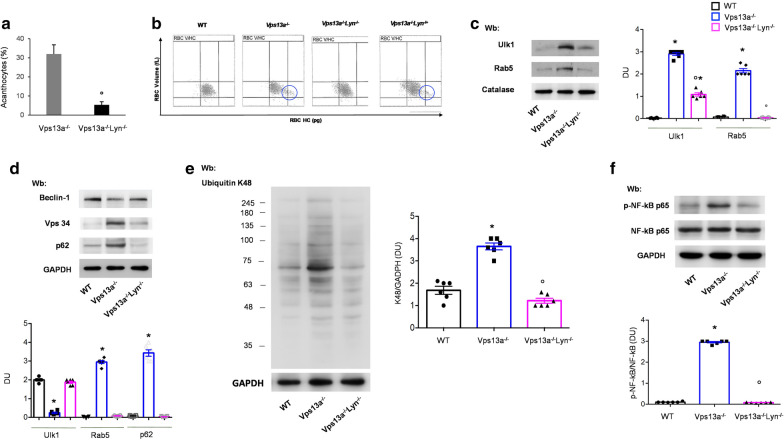
To further explore the role of Lyn in Vps13a−/− mice, we generated a Vps13a−/−Lyn−/− double knockout mouse (Fig. 3 and Additional file 1: Fig. S6, S7). Since Lyn−/− mice have been previously characterized and little role for Lyn kinase has been documented on primary neuronal functions [17, 18], we focused on the comparison between Vps13a−/− and Vps13a−/−Lyn−/− mice. In addition, the hematologic phenotype of Lyn−/− mice of age less than 15 months is similar to that of wild-type animals (Table S2) [25, 29]. In 12 months-old Vps13a−/− Lyn−/− mice, we observed significantly reduced acanthocyte numbers compared to either Vps13a−/− or to Vps13a−/−Lyn−/+ mice. This finding was accompanied by (1) disappearance of the dense red cell fraction; (2) the reduction in HDW and (3) the reduction of red cell osmotic fragility in Vps13a−/− Lyn−/−mice compared to both Vps13a−/− and Vps13a−/− Lyn−/+ mice (Fig. 3a, b, Additional file 1: Fig. S6A-B). Vps13a−/− mice genetically deficient in Lyn also exhibited normalization of the red cell content of autophagy-related proteins as compared to their elevated contents in Vps13a−/− mouse erythrocytes (Fig. 3c). In isolated basal ganglia from Vps13a−/− Lyn−/− mice, we found a slight but significant increase in beclin-1 and a marked reduction in accumulation of Vps34 and p62, supporting the dysregulated autophagy in mice lacking chorein by the incremental absence of Lyn (Fig. 3d). Indeed, we further observed significantly reduced accumulation of polyubiquitinated proteins in the basal ganglia from Vps13a−/− Lyn−/− mice compared to Vps13a−/− animals (Fig. 3e). This was associated with reduced NF-kB p65 activation, suggesting a reduction in neuroinflammation in Vps13a−/− Lyn−/− mice (Fig. 3f). Collectively these data support a key role of Lyn in disease mechanism of ChAc.
Fig. 3.
Vps13−/−Lyn−/− mice show amelioration of hematologic phenotype and improvement of autophagy and neuroinflammation in basal ganglia. a Quantitation of acanthocytes by brightfield microscopic analysis of peripheral blood smears from Vps13a−/− and Vps13a−/−Lyn−/− mice. Data from 50 visual fields was collected by two blinded researchers. Results are means ± SEM n = 6; °p < 0.002 versus Vps13a−/− by t-test b Left panel. Red cell distribution histograms generated for red blood cell volume (RBC Volume) and cell haemoglobin concentration (RBC-HC) of RBCs from wild-type (WT) control, Vps13a−/−, Vps13a−/−Lyn−/− and Vps13a−/−Lyn−/+ mice. One representative experiment of six with similar results is shown.The blue circle indicates the presence of a subpopulation of dense red cells containing acanthocytes, as described in human patients (Lupo et al. [42]). c Western blot (Wb) analysis of Ulk1 (Atg1) and Rab 5 from red cell cytosolic fractions of wild-type (WT), Vps13a−/− and Vps13a−/−Lyn−/− mice. Catalase was used as protein loading control. Densitometric analyses of the immunoblot bands similar to those shown are presented at right. Data are means ± SEM (n = 6; *P < 0.02 vs. WT; °P < 0.05 vs. Vps13a−/− by two-way-ANOVA/Bonferroni’s multiple comparison test). d Western blot (Wb) analysis of Beclin-1, Vps34 and p62 in isolated basal ganglia of wild-type (WT), Vps13a−/− and Vps13a−/−Lyn−/− mice. GAPDH served as protein loading control. Densitometric analyses of the immunoblot bands similar to those shown are presented at right. Data are means ± SEM (n = 6; *p < 0.02 vs. WT; °p < 0.02 compared to Vps13a−/− mice by two-way-ANOVA/Bonferroni’s multiple comparison test). e Western blot (Wb) analysis of K48-ubiquitinated proteins in basal ganglia isolated from wild-type (WT), Vps13a−/− and Vps13a−/−Lyn−/− mice. GAPDH served as protein loading control. Densitometric analyses of the immunoblot bands similar to those shown are presented at right. Data are means ± SEM (n = 6; *p < 0.02 vs. WT; °p < 0.02 compared to Vps13a−/− mice by two-way-ANOVA/Bonferroni’s multiple comparison test). f Western blot (Wb) analysis of phospho-NF-kB p65 (P-NF-kB), NF-kB in isolated basal ganglia of wild-type (WT), Vps13a−/− and Vps13a−/−Lyn−/− mice. GAPDH served as protein loading control. Densitometric analyses of the immunoblot bands similar to those shown are presented at right. Data are means ± SEM (n = 6; *p < 0.02 vs. WT; °p < 0.02 compared to Vps13a−/− mice by two-way-ANOVA/Bonferroni’s multiple comparison test)
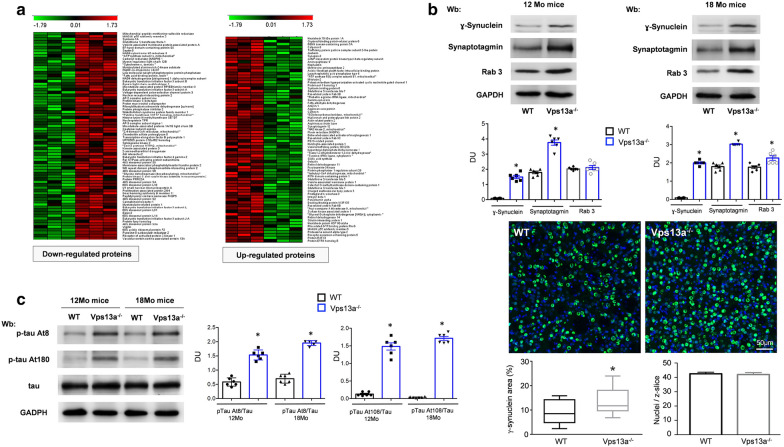
Proteomic analyses of Vps13a−/− mouse basal ganglia revealed abnormal proteostasis and accumulation of γ-synuclein
To understand the abnormal proteostasis in the basal ganglia of Vps13a−/− mice, we carried out proteomic analysis of Vps13a−/− mouse basal ganglia, using a label-free differential proteomic analysis based on Spectral Counts quantification, using Rsc method [75]. Among 3351 total identified proteins, 143 were selected as statistically significant changeable of which 69 were downregulated and 74 were upregulated compared to the basal ganglia of wild-type animals (Fig. 4a). Among the up-regulated proteins, we found of interest the following proteins for our model: (1) γ-Synuclein, member of the synuclein family; and (2) Synaptotagmins, which are involved in regulation of synaptic vesicle exocytosis; and (3) Syntaxin-1, involved in synaptic transmission [6, 30, 47]. We confirmed the increased expression of γ-Synuclein and synaptotagmin in isolated basal ganglia from 12- and 18- months old Vps13a−/− mice compared to wild-type animals (Fig. 4b). The accumulation of γ-synuclein was also confirmed by immunofluorescence microscopy (Fig. 4b, lower panel). The increased expression of γ-synuclein in basal ganglia from Vps13a−/− mice is of specific note since mice genetically over-expressing γ-synuclein display an age-dependent neurodegeneration, abnormal psycho-emotional status and motor deficiency [47]. Increased levels of γ-synuclein have been reported in cerebrospinal fluid in Alzheimer disease (AD) patients, in individuals with Creutzfeld-Jakob disease [48], and may contribute to the pathogenesis of amyotrophic lateral sclerosis (ALS) [52]. We then asked whether the increase cellular levels of γ-synuclein might be associated with the accumulation of other neurotoxic proteins such as neuronal microtubule-associated protein tau, which organizes into pathogenic fibrils in phosphorylated form. We analyzed the Tau phospho-epitopes At8 and At180, which are reported to have functional importance in the neurodegeneration of Alzheimer’s disease [33]. In basal ganglia from Vps13a−/− mice, we observed increased levels of At8 and At180 phosphorylated tau compared to wild-type animals (Fig. 4c). The accumulation of phosphorylated At8 and At180 tau protein has been previously linked with Lyn activity in the context of AD [18]: Remarkably, neither γ-synuclein nor tau phospho-epitopes At8 and 180 accumulated in isolated basal ganglia from Vps13a−/− Lyn−/− mice (Additional file 1: Fig. S7B-C). Taken together our evidence suggests ChAc as a novel disorder of proteostasis related to impaired autophagy with accumulation of neurotoxic proteins, supporting the rational to target active Lyn as a novel therapeutic option for ChAc.
Fig. 4.
Proteomic analysis of Vps13a−/− mouse basal ganglia revealed accumulation of neurotoxic proteins related to impaired autophagy. a Heatmaps of statistically relevant identified proteins. Each line corresponds to a protein and each column is relative to a different sample. The different coloration is dependent on quantity of protein present in sample based on the statistical performed analysis. Specifically, red color refers to up-regulated proteins while green is associated to down-regulated proteins. The logarithm Fold Change scale is also reported. Panel A and panel B report proteins down-regulated and up-regulated in Vps13a−/− mice compared to WT, respectively. b Upper panel. Western blot (Wb) analysis of γ-Synuclein, synaptotagmin and Rab 3 in isolated basal ganglia from 12 and 18 months (Mo) old wild-type and Vps13a−/− mice. GAPDH was the protein loading control. Middle panel. Densitometric analyses of the immunoblot bands similar to those shown are presented in bar graph. Data are means ± SEM (n = 6; * P < 0.02 ChAc vs. WT by t-test). Lower panel. Representative confocal images of the γ-synuclein protein (green) in the striatum of WT and Vps13a−/− mice at 18 months of age. Boxplots summarize the results presented as mean ± standard deviation (n = 3 animals for group; * P = 0.029). c Western blot (Wb) analysis of phopho-tau At8, At180, and total tau in isolated basal ganglia from 12 and 18 months (Mo) old wild-type and Vps13a−/− mice. GAPDH was the protein loading control. Densitometric analyses of the immunoblot bands similar to those shown are presented at right. Data are means ± SEM (n = 6; *p < 0.02 vs. WT by t-test)
Dasatinib ameliorates hematologic but not neurologic disease markers in Vps13a−/− mice
Since we tested dasatinib in our previous in vitro studies, we administrated dasatinib (50 mg/kg once a day for 4 weeks) to 12 months-old Vps13a−/− mice. Dasatinib-treated Vps13a−/− mice developed mild anemia associated with a significant reduction in reticulocyte count (Hb vehicle: 14.6 ± 0.8 vs. Hb dasatinib: 10.3 ± 1.5 g/dL; n = 8; p < 0.02; retic vehicle 390 ± 62 cell/µL vs. retic dasatinib: 201 ± 84 cell/µL n = 6; p < 0.05). In Vps13a−/− mouse RBCs, dasatinib markedly reduced the amount of active Lyn and improved autophagy as supported by the reduction of Ulk1 accumulation compared to vehicle treated animals (Additional file 1: Fig. S8A, B). However, basal ganglia of dasatinib-treated Vps13a−/− mice revealed no major difference in accumulation of active Lyn nor in levels of beclin-1 or beclin-1 related proteins such as Vps34 or Rab 5, suggesting a lacking effect of dasatinib in the central nervous system (Additional file 1: Fig. S8C, D). Mass spectrometric analyses with detection limit of 0.01 pg/µl detected no dasatinib in basal ganglia (Additional file 1: Fig. S9, Table S3). We therefore reviewed the literature on Lyn inhibitors that have been previously reported to better cross the BBB [12, 21, 28, 49, 67]. We focused on nilotinib, a second-generation TKI targeting Lyn, which has been previously shown to ameliorate mouse model phenotypes of PD and AD by improving autophagy [12, 21, 28], and has tested in 6 months phase II clinical trial in PD patients [49, 62].
Nilotinib reduces acanthocytosis, improves autophagy and neuroinflammation in basal ganglia from Vps13a−/− mice
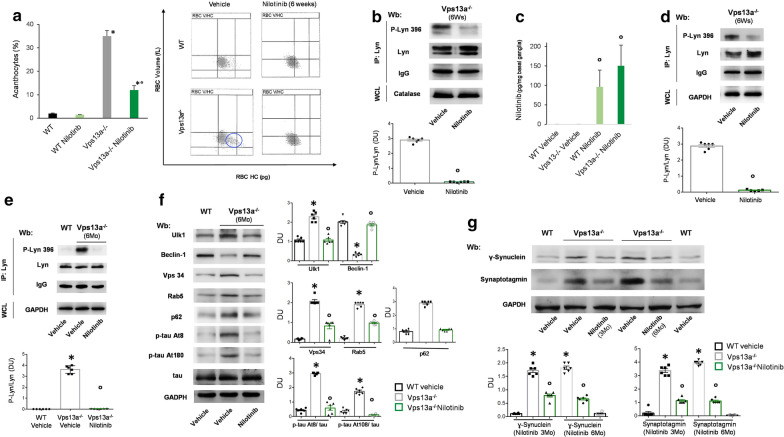
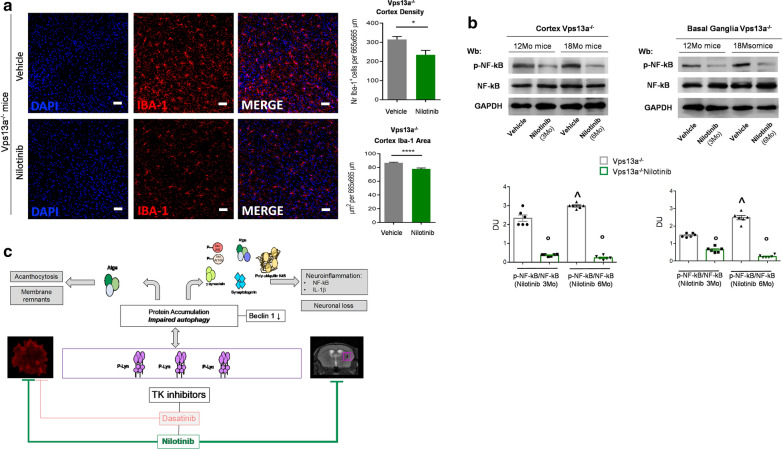
In Vps13a−/− mice, nilotinib (1) markedly reduced acanthocytosis and dense red cell fraction (Fig. 5a, Additional file 1: Fig. S10A); (2) prevented the accumulation of active Lyn in Vps13a−/− mouse RBCs (Fig. 5b); and (3) normalized RBC autophagy related proteins Ulk1 and Atg7 accumulation (Additional file 1: Fig. S10B). Nilotinib was detected by LC–MS/MS spectrometric analysis in basal ganglia from WT and Vps13a−/− mice (Fig. 5c, Additional file 1: Fig. S10C, Table S4). The presence of nilotinib in Vps13a−/− mouse basal ganglia was associated with markedly reduced basal ganglia accumulation of active Lyn after nilotinib treatment for 6 weeks or for 6 months (Fig. 5d, e). Accumulation of the key autophagy-related proteins Ulk1, Vps34, Rab5 and p62 were also reduced in basal ganglia of mice treated with nilotinib for either 6 weeks or 6 months (Fig. 5f, Additional file 1: Fig. S10D). Noteworthy, increased Vps13a−/− mouse basal ganglia beclin-1 levels were noted only after 6 months’ nilotinib treatment (Fig. 5f, Additional file 1: Fig. S10D). Accumulation of phospho-tau At8 and At180 (Fig. 5f, Additional file 1: Fig. S10E), γ-synuclein and synaptotagmin (Fig. 5g) were prevented by nilotinib treatment in basal ganglia from Vps13a−/− mice. Finally, we evaluated the impact of long-term treatment (3–6 months) on neuroinflammation in Vps13a−/− mice. As shown in Fig. 6a, b and Additional file 1: Fig. S11, we observed a significant reduction in microglia associated with decreased activated NF-kB p65 in both cortex and basal ganglia. Taken together, our data support the accumulation of active Lyn and impaired autophagy as possible therapeutic targets for clinical intervention in ChAc. We demonstrated that nilotinib crosses the BBB, improves autophagy and reduces neuroinflammation in a mouse model for ChAc.
Fig. 5.
Nilotinib ameliorates Vps13a−/− mouse red cell features and its passage into brain across the BBB prevents Lyn activation and improves autophagy. a Left panel. Quantitation of acanthocytes by brightfield microscopic analysis on Vps13a−/− and Vps13a−/− mice treated with nilotinib (25 mg/kg/d for 6 weeks). Data from 50 visual fields was collected by two blinded researchers. Results are means ± SEM n = 6; *P < 0.05 versus WT; °P < 0.05 versus vehicle treated Vps13a−/− by 2-way ANOVA with Bonferroni correction for multiple comparison. Right panel. Red cell distribution histograms generated for red blood cell volume (RBC Volume) and cell hemoglobin concentration (RBC-HC) of RBCs from wild-type (WT) control, Vps13a−/− mice treated with nilotinib (25 mg/kg/d for 6 weeks). One experiment representative of six others with similar result is shown. The blue circle indicates the presence of a subpopulation of dense red cells, containing acanthocytes as described in human patients (see also Lupo et al. [42]). b Total Lyn was immunoprecipitated from red cell cytosol fractions of Vps13a−/− mice treated with vehicle or with nilotinib (25 mg/kg/d for 6 weeks (6Ws)) and detected with antibody against active Lyn (phospho-Lyn 396) or antibody against total Lyn (Wb: Western-blot). The experiment shown is representative of 6 experiments. IgG is used as loading control as catalase in whole cell lysate (WCL). Lower panel. Densitometric analysis of the immunoblots; means ± SEM (n = 6; P < 0.05 vs. WT by t-test). c Quantification of nilotinib in isolated basal ganglia from wild-type (WT) and Vps13a−/− mice treated either with vehicle or nilotinib. Data are means ± SD (n = 6; °P < 0.05 vs. vehicle treated Vps13a−/− by 2-way ANOVA for multiple comparison). d Total Lyn was immunoprecipitated from basal ganglia of Vps13a−/− mice treated with vehicle or with nilotinib (25 mg/kg/d for 6 weeks (6Ws)). The experiment shown is representative of 6 experiments, each from an individual Vps13a−/− mouse and each with similar results. IgG and catalase are used as loading control. WCL: whole cell lysate. Lower panel. Densitometric analysis of the immunoblots; means ± SEM (n = 6; °P < 0.05 vs. WT by t-test). e Total Lyn was immunoprecipitated from basal ganglia of wild-type and Vps13a−/− mice treated with vehicle or with nilotinib (25 mg/kg/d for 6 months (6Mo), 12 months old mice) and detected with antibody against active Lyn (phospho-Lyn 396) or antibody against total Lyn (Wb: Western-blot). The experiment shown is representative of 6 experiments, each from an individual Vps13a−/− mouse and each with similar results. IgG is shown as loading control as well as GAPDH in whole cell lysate (WCL). Lower panel. Densitometric analysis of the immunoblots; means ± SEM (n = 6; *P < 0.05 vs. WT; °P < 0.05 vs. vehicle treated Vps13a−/− by 2-way ANOVA with Bonferroni correction for multiple comparison). f Western blot (Wb) analysis of Ulk1 (Atg1), Beclin-1, Vps34, Rab5, p62, phospho-tau At8, and At180 and total tau in isolated basal ganglia from 18 months old wild-type, and Vps13a−/− mice treated with either vehicle or nilotinib (25 mg/kg/d for 6 months (6Mo)). GAPDH was used as loading control (See Additional file 1: Fig. 14S for data on nilotinib treated 12 months-old mice). Right panel. Densitometric analyses of the immunoblot bands similar to those shown are presented at right. Data are means ± SEM (n = 6; *P < 0.05 vs. WT; °P < 0.05 vs. vehicle treated Vps13a−/− by 2-way ANOVA with Bonferroni correction for multiple comparison). g Western blot (Wb) analysis of γ-Synuclein and Synaptotagmin in isolated basal ganglia from 12 months old wild-type, and Vps13a−/− mice treated with either vehicle or nilotinib (25 mg/kg/d for 3 months (3Mo)) and 18 months old wild-type, and Vps13a−/− mice treated with either vehicle or nilotinib (25 mg/kg/d for 6 months (6Mo)). GAPDH was used as loading control. Lower panel. Densitometric analyses of the immunoblot bands similar to those shown are presented. Data are means ± SEM (n = 6; *P < 0.05 vs. WT; °P < 0.05 vs. vehicle treated Vps13a−/− by 2-way ANOVA with Bonferroni correction for multiple comparison)
Fig. 6.
Nilotinib decreases neuroinflammation in Vps13a−/− mice. a Representative images of Iba-1 positive microglia cells in cortex of Vps13a−/− mice treated with vehicle or with nilotinib (25 mg/kg/d for 6 months) (Microglia in red, nuclei in blue). Scale bar:50 mm. Quantitative analyses show significant differences in microglial density and activation in the cortex of Vps13a−/− vehicle compared to treated mice. Results are expressed as mean ± SEM (*P < 0.05; ****P < 0.0001; Unpaired t-test). b Western blot (Wb) analysis of phospho-NF-kB p65 and NF-kB p65 in the cortex (left panel) and in isolated basal ganglia (right panel) from 12 and 18 months (Mo) old wild-type mice and Vps13a−/− animals treated with vehicle or with nilotinib (25 mg/kg/d for 3 months (3Mo) and 6 months (6Mo) respectively). GAPDH was the protein loading control. Lower panel. Densitometric analyses of the immunoblot bands similar to those shown are presented. Data are means ± SEM (n = 6; ^P < 0.05 vs. 12 months old mice; °P < 0.05 vs. vehicle treated Vps13a−/− by 2-way ANOVA with Bonferroni correction for multiple comparison). c Mice genetically lacking chorein (Vps13a−/−) display phenotype similar to patients with chorea-acanthocytosis (ChAc). We show protein accumulation and impaired autophagy in both red cells and basal ganglia from Vps13a−/− mice. This is associated with neuronal loss, neuroinflammation and generation of circulating acanthocytes. Tyrosine kinase inhibitors (TKI) targeting Lyn kinase have been tested in Vps13a−/− mice. Nilotinib but not dasatinib reduces protein accumulation and ameliorates autophagy with reduction in neuronal loss and neuroinflammation as well as in circulating acanthocytes. Atgs: autophagy related proteins
Discussion
ChAc is a rare, progressive, multisystem neurodegenerative disease of young adulthood with no available treatment to halt or retard its devastating progression out. The identification of new therapeutic option(s) targeting disease mechanism(s) represents an urgent unmet need in ChAc. We first characterized Vps13a−/− mice in order to advance understanding of ChAc and to search for additional drug targets and novel drug candidates. Vps13a−/− mice display (1) acanthocytes; (2) signs of both hyper- and hypokinetic movement disorders; (3) accumulation of active Lyn and of autophagy-related proteins in RBCs; and (4) RBC retention of remnants of double membrane and multivesicular bodies. Abnormalities in motor behavior observed in Vps13a−/− mice correlate with the movement disorders, specifically dystonia, seen in ChAc patients [50, 70]. Indeed, the neurologic phenotype of Vps13a−/− mice resembles that of another mouse model for ChAc carrying a deletion of exons 60–61. [66]. In isolated basal ganglia of Vps13a−/− mice, we found signs of neurodegeneration associated with (1) accumulation of Lyn, stabilized in high molecular weight complexes; (2) accumulation of autophagy related proteins; and (3) reduction in expression of beclin-1, a key initiator of autophagy, due to increased caspase 3 activity. Normalization of phenotypes in the Vps13a−/−Lyn−/− double knock out model substantiates the central role of accumulation of active Lyn in the pathophysiology of ChAc. Lyn has been previously reported to contribute to varied neuronal functions throughout the phosphorylation of key substrates such as NMDA or AMPA receptors [16, 41, 59]. In addition, in vitro studies also suggest that active Lyn might reduce or alter exocytosis by phosphorylation of both proteins of synaptic vesicles and actin cytoskeleton [16]. Lyn and Src family kinases have been also shown to participate in autophagy by targeting various autophagy-related proteins such as Ulk1 [36, 37, 42]. However, the accumulation of active Src family kinases might be per se cytotoxic, contributing to impaired autophagy as reported in cancer cells [72, 73] The accumulation of At8- and At180-phosphorylated tau proteins and γ-synuclein as well as of polyubiquinated proteins in Vps13a−/− mouse basal ganglia is consistent with abnormal autophagy in the absence of chorein. Our data adds ChAc, for the first time, to the group of classical neurodegenerative proteinopathies such as AD, PD and HD. These three diseases are also characterized by abnormalities in autophagy as well as by reduced beclin-1 [58, 61, 63]. In addition, recent reports of severe neurodegeneration and impairment of autophagy in beclin1−/− mice further links beclin-1-dependent autophagy to neurodegenerative diseases [45, 55, 65]. In particular, the beclin-1-Vps34 complex is critical for autophagosome formation, subsequently involving Atg14 with recruitment of Rab 5 [45, 58]. In Vps13a−/− mice, the absence of chorein results in impairment of beclin-1 pathway with accumulation of Vps34, Atg14 and Rab5, suggesting a perturbation of protein trafficking associated with possible abnormalities in maturation and/or degradation of autophagosomes. This is further supported by the association between chorein with beclin-1 observed in basal ganglia from wild-type mice. Although chorein does not contain a recognized actin-binding motif, it carries a coil-coil binding motif that may be involved in beclin-1 interactions. A complex connection has been reported between beclin-1 and inflammation [24, 46]. In vitro studies show that block of autophagy results in up-regulation of pro-inflammatory cytokines such as IL-1β [24, 46]. Here, we found neuroinflammation in Vps13a−/− mice associated with activation of NF-kB p65 and increased expression of IL-1β further emphasizing similarities between ChAc and other neurodegenerative disorders characterized by abnormal proteostasis such as PD or AD [15]. At this stage we cannot determine whether neuroinflammation is directly involved in the etiology of ChAc or indirectly related to the neurodegenerative processes in the basal ganglia. Although systematic investigation of ChAc neuropathology in human brains is still lacking, preliminary reports on brain from patients with ChAc suggests the presence of microgliosis [38, 39, 50].
Collectively these findings led us to test in our mouse ChAc model a Lyn kinase inhibitor that crosses the BBB more easily than dasatinib. Nilotinib beneficially impacts ChAc mouse hematological phenotype and improves ChAc RBC features. Furthermore, nilotinib was detected in basal ganglia from Vps13a−/− mice resulting in improvement of autophagy with reduction of active Lyn and accumulation of autophagy related proteins. Vps13a−/− mice treated long-term with nilotinib exhibited increased levels of basal ganglia beclin-1, associated with reduced microglia density and reduced active NF-kB p65. These data further supported the link between beclin-1 dependent autophagy and inflammation in ChAc mice.
In conclusion, our data show for the first time that the pathogenesis of ChAc is linked to perturbation of beclin-1 pathway, resulting in impaired autophagy with accumulation of active Lyn and classic neurotoxic proteins such as γ-synuclein or phospho-tau At8 and At180. Abnormal autophagy contributes to NF-kB activation and expression of pro-inflammatory cytokines, such as Il-1b, in combination with microglia activation, sustaining a neuroinflammatory environment in ChAc as in other neurodegenerative disorders such as PD or AD. Our data prove active Lyn to be a key regulator of neurodegeneration in ChAc, thus generating a rationale to consider TKIs targeting Lyn per se as possible and safe novel therapeutic approach for ChAc patients (Fig. 6c). Our results propose BBB-permeable Lyn kinase inhibitors such as nilotinib as first-line treatment options for patients suffering from ChAc. As nilotinib is already in clinical use for treatment of other diseases [21], larger-scale studies of nilotinib for treatment of ChAc should be eligible for accelerated approval. Our data support the proposal to repurpose nilotinib as new therapeutic option for ChAc.
Supplementary Information
Acknowledgements
We thank Dr. Francesca Lupo (University of Verona), Dr. Manuela Medelin (University of Verona) for generation of preliminary data, analysis of some immunofluorescence images and fruitful discussion, respectively. We would like to thank CIRSAL, LURM and CPT (University of Verona) for technical support.
Authors' contributions
K.P. A.H. and L.D.F. designed all of the experiments. E.C.P. and G.C. contributed to the obtainment of neuropathology and behavior data. ET, PD generated mice lines, B.K., A.H. and L.D.F. supervised the project, K.P., A.H. and L.D.F. wrote the manuscript and all other authors critically revised the manuscript. K.P., A.H. and L.D.F.: Research project: Conception, Organization, Execution; Statistical Analysis: Design/Execution; Manuscript: Writing of the first draft. E.F. and A.M.: Research project: Conception, Organization, Execution. H.G.: Research project: Organization/Execution; Statistical Analysis: Design, Execution, Review and Critique; Manuscript: Review and Critique. G.C. and P.F.F.: Research project: Conception; Statistical Analysis: Design, Execution, Review and Critique; Manuscript: Writing of the first draft. E.C.P., F.D.G., A.I., F.C., F.G., I.A. and E.L.: Research project: Execution; Statistical Analysis: Execution. P.D.: Research project: Conception, Organization, Execution; Manuscript: Writing of the first draft. E.T. and P.P.: Research project: Conception; Manuscript: Writing of the first draft. A.A., M.M. and E.T.: Research project: Conception, Organization; Statistical Analysis: Design, Execution, Review and Critique; Manuscript: Writing of the first draft. SLALP, KA: Research project: Execution; Statistical Analysis: Execution; Manuscript: Review and Critique. T.Z.: Manuscript: Review and Critique. R.O.: Research project: Conception/Organization; Manuscript: Review and Critique. F.L.: Research project: Organization; Manuscript: Review and Critique. A.M.B.: Research project: Conception; Statistical Analysis: Review and Critique; Manuscript: Writing of the first draft. E.T.: Research project: Conception, Organization, Execution; Statistical Analysis: Execution. Aio, M.B., A.D. and R.H.W.: Manuscript: Writing of the first draft. M.B.: Research project: Conception, Organization, Execution; Statistical Analysis: Review and Critique; Manuscript: Writing of the first draft. A.S.: Research project: Execution. All authors read and approved the final manuscript.
Funding
This study was supported in part by the Centre for Regenerative Therapies Dresden (CRTD), the German Center for Neurodegenerative Diseases (DZNE), research site Dresden, the Helmholtz Virtual Institute (VH-VI-510), by the Advocacy for Neuroacanthocytosis Patients (AH, LDF), by the Else Kröner Clinician Scientist Program (TU Dresden, Germany) and the Rostock Academy for Clinician Scientists (RACS, University of Rostock, Germany) (KP), the “Hermann und Lilly Schilling-Stiftung für medizinische Forschung im Stifterverband” (AH). The work was also supported in part by the European Research Council (ERC) Advanced Grant Immunoalzheimer #695714 (to GC) and FUR-UNIVR (LDF).
Declarations
Competing interests
KP has received funding from the Else Kröner Clinician Scientist Program (TU Dresden, Germany) and the MeDDrive grant of the Technische Universität Dresden, and the Rostock Academy for Clinician Scientists (RACS, University of Rostock, Germany). HG, LP, AM, FL, EF, GC, ECP RO, PFF, PDF, ET, FDG, PP, AA, AI, FC, MM, FG, ET, AMB, ET, IA, AI, MB, EL, AS, MB, AD AND RHW none. KA received honoraria for presentation and consulting service from Biogen Idec, Merck, Sanofi and Roche. TZ received honoraria for presentation and consulting service from Biogen, Bayer, Celgene, Novartis, Roche, Sanofi, Teva. He received additional financial support for research activities from BAT, Biogen, Novartis, Roche, Teva, and Sanofi Aventis. AH has received funding from the Federal Ministry of Education and Research (BMBF), the Helmholtz-Association, the Schilling-Stiftung, the “Innovationsfond des Gemeinsamen Bundenausschusses”. He has received honoraria for presentations, advisory boards, consultations from BIOGEN and Desitin. He has received royalties from Elsevier Press. He serves as an editorial board member of BMC Neurology. SLA has received research funding from the US DoD and Quest Diagnostics. He has received consultation fees from the Broad Institute, the Medical University of Vienna, and Quest Diagnostics. LDF has received research funding by Agios.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kevin Peikert, Enrica Federti, Andreas Hermann and Lucia De Franceschi have contributed equally to this work
Contributor Information
Andreas Hermann, Email: Andreas.Hermann@med.uni-rostock.de.
Lucia De Franceschi, Email: lucia.defranceschi@univr.it.
References
- 1.Arora A, Bhagat N. Insight into the molecular imaging of Alzheimer's disease. Int J Biomed Imaging. 2016;2016:7462014. doi: 10.1155/2016/7462014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baiguera C, Alghisi M, Pinna A, Bellucci A, De Luca MA, Frau L, Morelli M, Ingrassia R, Benarese M, Porrini V, Pellitteri M, Bertini G, Fabene PF, Sigala S, Spillantini MG, Liou HC, Spano PF, Pizzi M. Late-onset Parkinsonism in NFkappaB/c-Rel-deficient mice. Brain. 2012;135:2750–2765. doi: 10.1093/brain/aws193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batka RJ, Brown TJ, Mcmillan KP, Meadows RM, Jones KJ, Haulcomb MM. The need for speed in rodent locomotion analyses. Anat Rec (Hoboken) 2014;297:1839–1864. doi: 10.1002/ar.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blandini F, Cosentino M, Mangiagalli A, Marino F, Samuele A, Rasini E, Fancellu R, Tassorelli C, Pacchetti C, Martignoni E, Riboldazzi G, Calandrella D, Lecchini S, Frigo G, Nappi G. Modifications of apoptosis-related protein levels in lymphocytes of patients with Parkinson's disease. The effect of dopaminergic treatment. J Neural Transm (Vienna) 2004;111:1017–1030. doi: 10.1007/s00702-004-0123-1. [DOI] [PubMed] [Google Scholar]
- 5.Clark MR, Aminoff MJ, Chiu DT, Kuypers FA, Friend DS. Red cell deformability and lipid composition in two forms of acanthocytosis: enrichment of acanthocytic populations by density gradient centrifugation. J Lab Clin Med. 1989;113:469–481. [PubMed] [Google Scholar]
- 6.Colacurcio DJ, Pensalfini A, Jiang Y, Nixon RA. Dysfunction of autophagy and endosomal-lysosomal pathways: roles in pathogenesis of down syndrome and Alzheimer's disease. Free Radic Biol Med. 2018;114:40–51. doi: 10.1016/j.freeradbiomed.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Critchley EM, Clark DB, Wikler A. Acanthocytosis and neurological disorder without betalipoproteinemia. Arch Neurol. 1968;18:134–140. doi: 10.1001/archneur.1968.00470320036004. [DOI] [PubMed] [Google Scholar]
- 8.Danek A, Bader B, Velayos-Baeza A, Walker RH. Autosomal recessive transmission of chorea-acanthocytosis confirmed. Acta Neuropathol. 2012;123:905–906. doi: 10.1007/s00401-012-0971-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Franceschi L, Tomelleri C, Matte A, Brunati AM, Bovee-Geurts PH, Bertoldi M, Lasonder E, Tibaldi E, Danek A, Walker RH, Jung HH, Bader B, Siciliano A, Ferru E, Mohandas N, Bosman GJ. Erythrocyte membrane changes of chorea-acanthocytosis are the result of altered Lyn kinase activity. Blood. 2011;118:5652–5663. doi: 10.1182/blood-2011-05-355339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobson-Stone C, Danek A, Rampoldi L, Hardie RJ, Chalmers RM, Wood NW, Bohlega S, Dotti MT, Federico A, Shizuka M, Tanaka M, Watanabe M, Ikeda Y, Brin M, Goldfarb LG, Karp BI, Mohiddin S, Fananapazir L, Storch A, Fryer AE, Maddison P, Sibon I, Trevisol-Bittencourt PC, Singer C, Caballero IR, Aasly JO, Schmierer K, Dengler R, Hiersemenzel LP, Zeviani M, Meiner V, Lossos A, Johnson S, Mercado FC, Sorrentino G, Dupre N, Rouleau GA, Volkmann J, Arpa J, Lees A, Geraud G, Chouinard S, Nemeth A, Monaco AP. Mutational spectrum of the CHAC gene in patients with chorea-acanthocytosis. Eur J Hum Genet. 2002;10:773–781. doi: 10.1038/sj.ejhg.5200866. [DOI] [PubMed] [Google Scholar]
- 11.Duarte JM, Do KQ, Gruetter R. Longitudinal neurochemical modifications in the aging mouse brain measured in vivo by 1H magnetic resonance spectroscopy. Neurobiol Aging. 2014;35:1660–1668. doi: 10.1016/j.neurobiolaging.2014.01.135. [DOI] [PubMed] [Google Scholar]
- 12.Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS. Emerging therapies in Parkinson disease: repurposed drugs and new approaches. Nat Rev Neurol. 2019;15:204–223. doi: 10.1038/s41582-019-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estevez-Fraga C, Lopez-Sendon Moreno JL, Martinez-Castrillo JC. Phenomenology and disease progression of chorea-acanthocytosis patients in Spain. Parkinsonism Relat Disord. 2018;49:17–21. doi: 10.1016/j.parkreldis.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Gao M, Yang H. VPS13: a lipid transfer protein making contacts at multiple cellular locations. J Cell Biol. 2018;217:3322–3324. doi: 10.1083/jcb.201808151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelders G, Baekelandt V, Van der Perren A. Linking neuroinflammation and neurodegeneration in Parkinson's disease. J Immunol Res. 2018;2018:4784268. doi: 10.1155/2018/4784268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibb SL, Jeanblanc J, Barak S, Yowell QV, Yaka R, Ron D. Lyn kinase regulates mesolimbic dopamine release: implication for alcohol reward. J Neurosci. 2011;31:2180–2187. doi: 10.1523/JNEUROSCI.5540-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwon Y, Kim SH, Kim HT, Kam TI, Park J, Lim B, Cha H, Chang HJ, Hong YR, Jung YK. Amelioration of amyloid beta-FcgammaRIIb neurotoxicity and tau pathologies by targeting LYN. FASEB J Off Publ Fed Am Soc Exp Biol. 2019;33:4300–4313. doi: 10.1096/fj.201800926R. [DOI] [PubMed] [Google Scholar]
- 18.Gwon Y, Kim SH, Kim HT, Kam TI, Park J, Lim B, Cha H, Chang HJ, Hong YR, Jung YK. Amelioration of amyloid β-FcγRIIb neurotoxicity and tau pathologies by targeting LYN. FASEB J. 2019;33:4300–4313. doi: 10.1096/fj.201800926R. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC. Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci USA. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hebron ML, Lonskaya I, Moussa CE. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of alpha-synuclein in Parkinson's disease models. Hum Mol Genet. 2013;22:3315–3328. doi: 10.1093/hmg/ddt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heffron TP. Small molecule kinase inhibitors for the treatment of brain cancer. J Med Chem. 2016;59:10030–10066. doi: 10.1021/acs.jmedchem.6b00618. [DOI] [PubMed] [Google Scholar]
- 22.Hermann A, Walker RH. Diagnosis and treatment of chorea syndromes. Curr Neurol Neurosci Rep. 2015;15:514. doi: 10.1007/s11910-014-0514-0. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 24.Houtman J, Freitag K, Gimber N, Schmoranzer J, Heppner FL, Jendrach M. Beclin1-driven autophagy modulates the inflammatory response of microglia via NLRP3. EMBO J. 2019 doi: 10.15252/embj.201899430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingley E, McCarthy DJ, Pore JR, Sarna MK, Adenan AS, Wright MJ, Erber W, Tilbrook PA, Klinken SP. Lyn deficiency reduces GATA-1, EKLF and STAT5, and induces extramedullary stress erythropoiesis. Oncogene. 2005;24:336–343. doi: 10.1038/sj.onc.1208199. [DOI] [PubMed] [Google Scholar]
- 26.Jung HH, Danek A, Walker RH. Neuroacanthocytosis syndromes. Orphanet J Rare Dis. 2011;6:68. doi: 10.1186/1750-1172-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalish BT, Matte A, Andolfo I, Iolascon A, Weinberg O, Ghigo A, Cimino J, Siciliano A, Hirsch E, Federti E, Puder M, Brugnara C, De Franceschi L. Dietary ω-3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease. Haematologica. 2015;100:870–880. doi: 10.3324/haematol.2015.124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karuppagounder SS, Brahmachari S, Lee Y, Dawson VL, Dawson TM, Ko HS. The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson's disease. Sci Rep. 2014;4:4874. doi: 10.1038/srep04874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karur VG, Lowell CA, Besmer P, Agosti V, Wojchowski DM. Lyn kinase promotes erythroblast expansion and late-stage development. Blood. 2006;108:1524–1532. doi: 10.1182/blood-2005-09-008243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura N, Yanagisawa K. Traffic jam hypothesis: Relationship between endocytic dysfunction and Alzheimer's disease. Neurochem Int. 2018;119:35–41. doi: 10.1016/j.neuint.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Kuhla A, Ruhlmann C, Lindner T, Polei S, Hadlich S, Krause BJ, Vollmar B, Teipel SJ. APPswe/PS1dE9 mice with cortical amyloid pathology show a reduced NAA/Cr ratio without apparent brain atrophy: a MRS and MRI study. NeuroImage Clin. 2017;15:581–586. doi: 10.1016/j.nicl.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar N, Leonzino M, Hancock-Cerutti W, Horenkamp FA, Li P, Lees JA, Wheeler H, Reinisch KM, De Camilli P. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol. 2018;217:3625–3639. doi: 10.1083/jcb.201807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landrieu I, Smet-Nocca C, Amniai L, Louis JV, Wieruszeski JM, Goris J, Janssens V, Lippens G. Molecular implication of PP2A and Pin1 in the Alzheimer's disease specific hyperphosphorylation of Tau. PLoS ONE. 2011;6:e21521. doi: 10.1371/journal.pone.0021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lesage S, Drouet V, Majounie E, Deramecourt V, Jacoupy M, Nicolas A, Cormier-Dequaire F, Hassoun SM, Pujol C, Ciura S, Erpapazoglou Z, Usenko T, Maurage CA, Sahbatou M, Liebau S, Ding J, Bilgic B, Emre M, Erginel-Unaltuna N, Guven G, Tison F, Tranchant C, Vidailhet M, Corvol JC, Krack P, Leutenegger AL, Nalls MA, Hernandez DG, Heutink P, Gibbs JR, Hardy J, Wood NW, Gasser T, Durr A, Deleuze JF, Tazir M, Destee A, Lohmann E, Kabashi E, Singleton A, Corti O, Brice A. Loss of VPS13C function in autosomal-recessive parkinsonism causes mitochondrial dysfunction and increases PINK1/Parkin-dependent mitophagy. Am J Hum Genet. 2016;98:500–513. doi: 10.1016/j.ajhg.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine IM, Estes JW, Looney JM. Hereditary neurological disease with acanthocytosis. A new syndrome. Arch Neurol. 1968;19:403–409. doi: 10.1001/archneur.1968.00480040069007. [DOI] [PubMed] [Google Scholar]
- 36.Li X, He S, Zhou X, Ye Y, Tan S, Zhang S, Li R, Yu M, Jundt MC, Hidebrand A, Wang Y, Li G, Huang C, Wu M. Lyn delivers bacteria to lysosomes for eradication through TLR2-initiated autophagy related phagocytosis. PLoS Pathog. 2016;12:e1005363. doi: 10.1371/journal.ppat.1005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichtenstein A, Minogue PJ, Beyer EC, Berthoud VM. Autophagy: a pathway that contributes to connexin degradation. J Cell Sci. 2011;124:910–920. doi: 10.1242/jcs.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Heinsen H, Grinberg LT, Alho E, Amaro E, Jr, Pasqualucci CA, Rub U, den Dunnen W, Arzberger T, Schmitz C, Kiessling M, Bader B, Danek A. Subcortical neurodegeneration in chorea: similarities and differences between chorea-acanthocytosis and Huntington's disease. Parkinsonism Relat Disord. 2018;49:54–59. doi: 10.1016/j.parkreldis.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Heinsen H, Grinberg LT, Alho E, Amaro E, Jr, Pasqualucci CA, Rub U, Seidel K, den Dunnen W, Arzberger T, Schmitz C, Kiessling MC, Bader B, Danek A. Pathoarchitectonics of the cerebral cortex in chorea-acanthocytosis and HD. Neuropathol Appl Neurobiol. 2018 doi: 10.1111/nan.12495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lonskaya I, Hebron ML, Desforges NM, Franjie A, Moussa CE. Tyrosine kinase inhibition increases functional parkin-Beclin-1 interaction and enhances amyloid clearance and cognitive performance. EMBO Mol Med. 2013;5:1247–1262. doi: 10.1002/emmm.201302771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu W, Fang W, Li J, Zhang B, Yang Q, Yan X, Peng L, Ai H, Wang JJ, Liu X, Luo J, Yang W. Phosphorylation of tyrosine 1070 at the GluN2B subunit is regulated by synaptic activity and critical for surface expression of N-methyl-D-aspartate (NMDA) receptors. J Biol Chem. 2015;290:22945–22954. doi: 10.1074/jbc.M115.663450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lupo F, Tibaldi E, Matte A, Sharma AK, Brunati AM, Alper SL, Zancanaro C, Benati D, Siciliano A, Bertoldi M, Zonta F, Storch A, Walker RH, Danek A, Bader B, Hermann A, De Franceschi L. A new molecular link between defective autophagy and erythroid abnormalities in chorea-acanthocytosis. Blood. 2016;128:2976–2987. doi: 10.1182/blood-2016-07-727321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matte A, Federti E, Winter M, Koerner A, Harmeier A, Mazer N, Tomka T, Di Paolo ML, De Falco L, Andolfo I, Beneduce E, Iolascon A, Macias-Garcia A, Chen JJ, Janin A, Lebouef C, Turrini F, Brugnara C, De Franceschi L. Bitopertin, a selective oral GLYT1 inhibitor, improves anemia in a mouse model of β-thalassemia. JCI Insight. 2019 doi: 10.1172/jci.insight.130111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matte A, Low PS, Turrini F, Bertoldi M, Campanella ME, Spano D, Pantaleo A, Siciliano A, De Franceschi L. Peroxiredoxin-2 expression is increased in beta-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic Biol Med. 2010;49:457–466. doi: 10.1016/j.freeradbiomed.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKnight NC, Zhong Y, Wold MS, Gong S, Phillips GR, Dou Z, Zhao Y, Heintz N, Zong WX, Yue Z. Beclin 1 is required for neuron viability and regulates endosome pathways via the UVRAG-VPS34 complex. PLoS Genet. 2014;10:e1004626. doi: 10.1371/journal.pgen.1004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mo Y, Sun YY, Liu KY. Autophagy and inflammation in ischemic stroke. Neural Regen Res. 2020;15:1388–1396. doi: 10.4103/1673-5374.274331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ninkina N, Peters O, Millership S, Salem H, van der Putten H, Buchman VL. Gamma-synucleinopathy: neurodegeneration associated with overexpression of the mouse protein. Hum Mol Genet. 2009;18:1779–1794. doi: 10.1093/hmg/ddp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oeckl P, Metzger F, Nagl M, von Arnim CA, Halbgebauer S, Steinacker P, Ludolph AC, Otto M. Alpha-, beta-, and gamma-synuclein quantification in cerebrospinal fluid by multiple reaction monitoring reveals increased concentrations in Alzheimer's and Creutzfeldt–Jakob disease but no alteration in synucleinopathies. Mol Cell Proteomics. 2016;15:3126–3138. doi: 10.1074/mcp.M116.059915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pagan FL, Hebron ML, Wilmarth B, Torres-Yaghi Y, Lawler A, Mundel EE, Yusuf N, Starr NJ, Anjum M, Arellano J, Howard HH, Shi W, Mulki S, Kurd-Misto T, Matar S, Liu X, Ahn J, Moussa C. Nilotinib effects on safety, tolerability, and potential biomarkers in Parkinson disease: a phase 2 randomized clinical trial. JAMA Neurol. 2019 doi: 10.1001/jamaneurol.2019.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peikert K, Danek A, Hermann A. Current state of knowledge in chorea-acanthocytosis as core neuroacanthocytosis syndrome. Eur J Med Genet. 2017 doi: 10.1016/j.ejmg.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 51.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters OM, Shelkovnikova T, Highley JR, Cooper-Knock J, Hortobagyi T, Troakes C, Ninkina N, Buchman VL. Gamma-synuclein pathology in amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2015;2:29–37. doi: 10.1002/acn3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rampoldi L, Danek A, Monaco AP. Clinical features and molecular bases of neuroacanthocytosis. J Mol Med (Berl) 2002;80:475–491. doi: 10.1007/s00109-002-0349-z. [DOI] [PubMed] [Google Scholar]
- 54.Rampoldi L, Dobson-Stone C, Rubio JP, Danek A, Chalmers RM, Wood NW, Verellen C, Ferrer X, Malandrini A, Fabrizi GM, Brown R, Vance J, Pericak-Vance M, Rudolf G, Carrè S, Alonso E, Manfredi M, Németh AH, Monaco AP. A conserved sorting-associated protein is mutant in chorea-acanthocytosis. Nat Genet. 2001;28:119. doi: 10.1038/88821. [DOI] [PubMed] [Google Scholar]
- 55.Ravikumar B, Imarisio S, Sarkar S, O'Kane CJ, Rubinsztein DC. Rab5 modulates aggregation and toxicity of mutant huntingtin through macroautophagy in cell and fly models of Huntington disease. J Cell Sci. 2008;121:1649–1660. doi: 10.1242/jcs.025726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res. 2014;24:42–57. doi: 10.1038/cr.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Impaired autophagy and APP processing in Alzheimer's disease: the potential role of Beclin 1 interactome. Prog Neurobiol. 2013;106–107:33–54. doi: 10.1016/j.pneurobio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 60.Schwenk BM, Hartmann H, Serdaroglu A, Schludi MH, Hornburg D, Meissner F, Orozco D, Colombo A, Tahirovic S, Michaelsen M, Schreiber F, Haupt S, Peitz M, Brustle O, Kupper C, Klopstock T, Otto M, Ludolph AC, Arzberger T, Kuhn PH, Edbauer D. TDP-43 loss of function inhibits endosomal trafficking and alters trophic signaling in neurons. EMBO J. 2016;35:2350–2370. doi: 10.15252/embj.201694221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shibata M, Lu T, Furuya T, Degterev A, Mizushima N, Yoshimori T, MacDonald M, Yankner B, Yuan J. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem. 2006;281:14474–14485. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- 62.Simuni T, Fiske B, Merchant K, Coffey CS, Klingner E, Caspell-Garcia C, Lafontant DE, Matthews H, Wyse RK, Brundin P, Simon DK, Schwarzschild M, Weiner D, Adams J, Venuto C, Dawson TM, Baker L, Kostrzebski M, Ward T, Rafaloff G, Parkinson Study Group N-PDI, Collaborators Efficacy of nilotinib in patients with moderately advanced Parkinson disease: a randomized clinical trial. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci Off J Soc Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanslowsky N, Reinhardt P, Glass H, Kalmbach N, Naujock M, Hensel N, Lubben V, Pal A, Venneri A, Lupo F, De Franceschi L, Claus P, Sterneckert J, Storch A, Hermann A, Wegner F. Neuronal dysfunction in iPSC-derived medium spiny neurons from chorea-acanthocytosis patients is reversed by Src kinase inhibition and F-actin stabilization. J Neurosci. 2016;36:12027–12043. doi: 10.1523/JNEUROSCI.0456-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 66.Tomemori Y, Ichiba M, Kusumoto A, Mizuno E, Sato D, Muroya S, Nakamura M, Kawaguchi H, Yoshida H, Ueno S, Nakao K, Nakamura K, Aiba A, Katsuki M, Sano A. A gene-targeted mouse model for chorea-acanthocytosis. J Neurochem. 2005;92:759–766. doi: 10.1111/j.1471-4159.2004.02924.x. [DOI] [PubMed] [Google Scholar]
- 67.Turner RS, Hebron ML, Lawler A, Mundel EE, Yusuf N, Starr JN, Anjum M, Pagan F, Torres-Yaghi Y, Shi W, Mulki S, Ferrante D, Matar S, Liu X, Esposito G, Berkowitz F, Jiang X, Ahn J, Moussa C. Nilotinib effects on safety, tolerability, and biomarkers in Alzheimer's disease. Ann Neurol. 2020;88:183–194. doi: 10.1002/ana.25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ueno S, Maruki Y, Nakamura M, Tomemori Y, Kamae K, Tanabe H, Yamashita Y, Matsuda S, Kaneko S, Sano A. The gene encoding a newly discovered protein, chorein, is mutated in chorea-acanthocytosis. Nat Genet. 2001;28:121. doi: 10.1038/88825. [DOI] [PubMed] [Google Scholar]
- 69.Vandeputte C, Taymans JM, Casteels C, Coun F, Ni Y, Van Laere K, Baekelandt V. Automated quantitative gait analysis in animal models of movement disorders. BMC Neurosci. 2010;11:92. doi: 10.1186/1471-2202-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Velayos Baeza A, Dobson-Stone C, Rampoldi L, Bader B, Walker RH, Danek A, Monaco AP, et al. Chorea-acanthocytosis. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews. Seattle: University of Washington; 1993. [Google Scholar]
- 71.Walker RH. Management of neuroacanthocytosis syndromes. Tremor Other Hyperkinetic Mov (New York, NY) 2015;5:346. doi: 10.7916/D8W66K48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Z, Chang PC, Yang JC, Chu CY, Wang LY, Chen NT, Ma AH, Desai SJ, Lo SH, Evans CP, Lam KS, Kung HJ. Autophagy blockade sensitizes prostate cancer cells towards Src family kinase inhibitors. Genes Cancer. 2010;1:40–49. doi: 10.1177/1947601909358324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 74.Yeshaw WM, van der Zwaag M, Pinto F, Lahaye LL, Faber AI, Gomez-Sanchez R, Dolga AM, Poland C, Monaco AP, van IJzendoorn SCD, Grzeschik NA, Velayos-Baeza A, Sibon OC. Human VPS13A is associated with multiple organelles and influences mitochondrial morphology and lipid droplet motility. eLife. 2019 doi: 10.7554/eLife.43561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu W, Smith JW, Huang CM. Mass spectrometry-based label-free quantitative proteomics. J Biomed Biotechnol. 2010;2010:840518. doi: 10.1155/2010/840518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.