Fig. 2. Mass spectrometry (MS) identification of S-nitrosylated Cys residue in Uch-L1 and atomic resolution model.
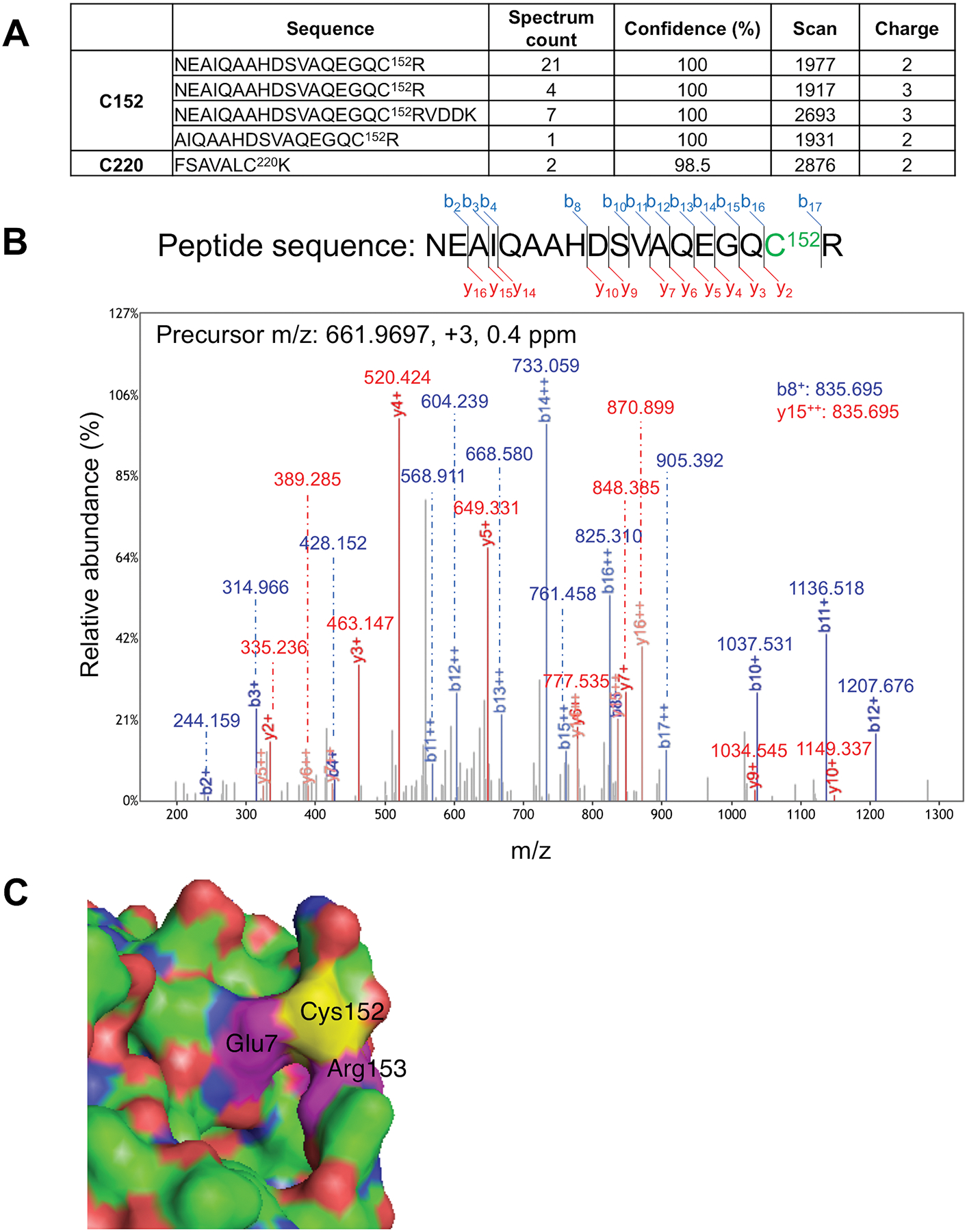
(A) Table summarizing S-nitrosylated peptides for Uch-L1 by MS analysis. Four peptides were identified containing Cys152, and one peptide with Cys220. Spectral counting clearly demonstrated that Cys152 is the predominant S-nitrosylation site on Uch-L1 (total spectral counts 33 for Cys152 vs. 2 for Cys220). (B) Tandem mass spectrum of a Uchl-L1 peptide identifying S-nitrosylation at Cys152. The measured mass, charge state, and measurement accuracy of the precursor ion are displayed. The b (blue) and y (red) fragment ions are annotated with the measured mass. (C) Crystal structure of Uch-L1 (PDB ID: 2ETL) near Cys152 (yellow). Magenta: Glu7 and Arg153. Molecular visualization and graphics handling were performed using PyMol.
