Fig. 6. Redox blot quantifying transnitrosylation from Uch-L1 to Cdk5.
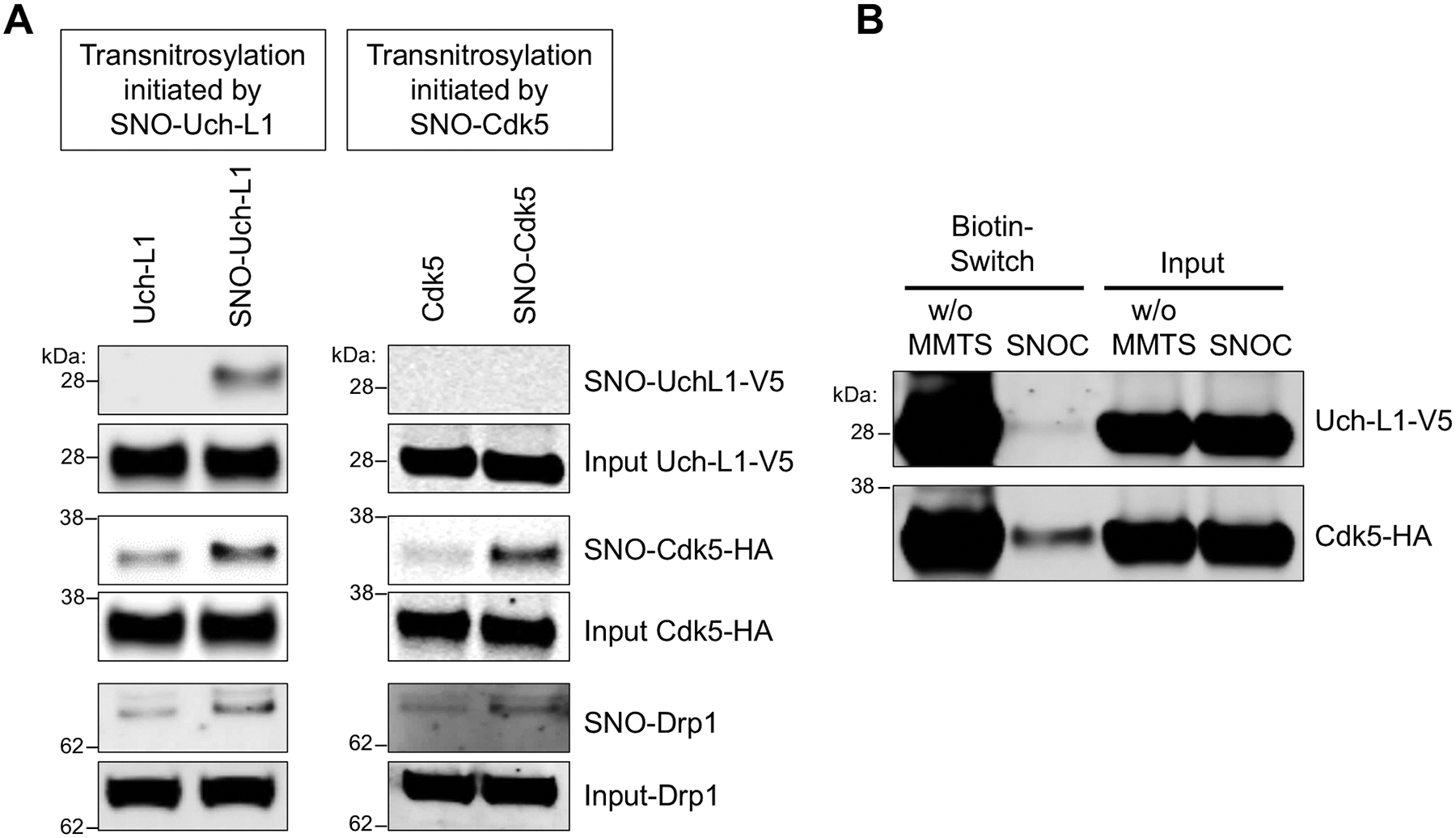
(A) Transnitrosylation occurs predominantly from SNO-Uch-L1 to Cdk5 to Drp1 rather than in the opposite direction from SNO-Cdk5 to Uch-L1. Expression of Uch-L1-V5 and Cdk5-HA increased the formation of SNO-Drp1, as evidenced on biotin-switch assay. Lysates of SH-SY5Y cells were prepared by V5- or HA-immunoprecipitation and exposed to 100 μM SNOC. These immunoprecipitates were then added to whole-cell lysates expressing either HA-Cdk5 or Uch-L1-V5, which were subjected to the biotin-switch assay to assess transnitrosylation. (B) SH-SY5Y cells were transfected with Uchl-L1-V5 and Cdk5-HA tagged constructs, and exposed to 50 μM SNOC at room temperature. After 30 min, cell lysates were prepared, incubated to achieve steady state (~1 h, by which time SNOC, a short-lived NO donor, had completely dissipated), and then subjected to the biotin-switch assay. Methyl-methanethiosulfonate (MMTS, 25 mM) was used to block free thiols during the assay for S-nitrosylated protein. Chemically-reduced thiol protein represents the relative amount of total protein obtained by the biotin-switch assay performed in the absence of MMTS (w/o MMTS). SNO-protein represents the relative amount of S-nitrosylated protein obtained by the biotin-switch assay. The relative concentration of protein in a redox pair in the S-nitrosylated (oxidized) form and the reduced form can be measured by quantitative densitometry of their respective bands on the gels (n = 4).
