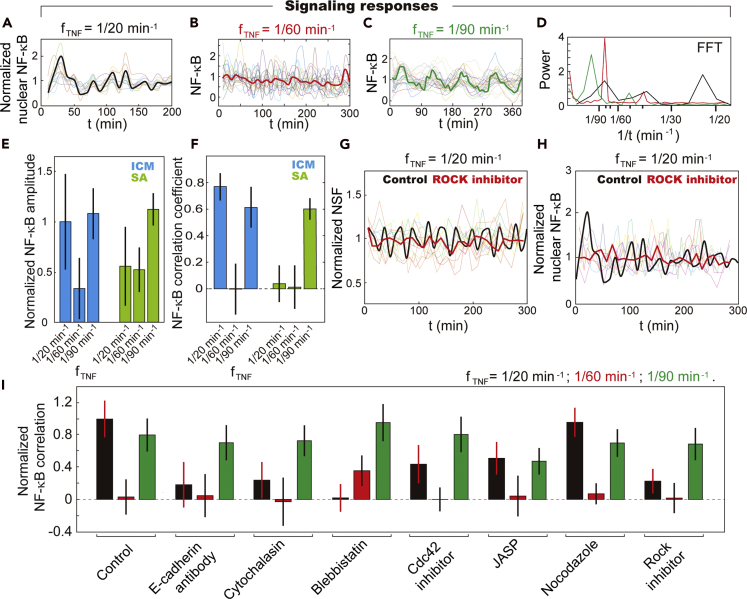
Figure 4.
Collective signaling response of population cells in ICM upon periodic TNF-α stimulation
(A–C) NF-κB dynamics of individual cells in ICM upon periodic TNF-α stimulation. NF-κB traces were normalized by the average value.
(D) Fast Fourier transform (FFT) shows dominant oscillation frequency close to NSF. In (A)-(C), the translucent lines are the traces of individual cells and the solid lines reflect the averaged value of single cells traces.
(E) NF-κB oscillation amplitude is maximized at 1/90 min−1, when getting entrained, and greatly enhanced in the ICM in response to 1/20 min−1 TNF-α stimulation as compared with SA cells. The NF-κB oscillation amplitude of individual cells under different conditions was normalized by value of the control samples, i.e., ICM treated by only 1/20 min−1 TNF-α. The error bars represent standard deviation of population cell's nuclear NF-κB fluctuation amplitude.
(F) Cross-correlation analysis of the signaling responses of population cells in the ICM reveals that the collective behavior is most obvious in NF-κB dynamics when stimulated by 1/20 and 1/90 min−1 TNF-α in ICM, and only at 1/90 min−1 TNF-α for SA cells. The error bars represent standard deviation of the correlation coefficients of population cells' NF-κB oscillation through NE.
(G and H) NF-κB and NSF traces of single fibroblasts in ICM treated with ROCK inhibitor, which is followed by 1/20 min−1 TNF-α stimulation. The NSF and NF-κB traces were normalized by their average values. In (G)-(H), the translucent lines are the traces of individual cells being treated by ROCK inhibitor and the solid lines reflect the averaged value of single cells traces. It is demonstrated that the application of ROCK inhibitor disrupts the collective activities of the ICM in NSF and NF-κB dynamics shown in Figures 1K and 4A.
(I) The collective signaling responses were disrupted by drugs, which disrupt collective NSF. The collective cellular responses at 1/90 min−1 were unaffected. The NF-κB correlation coefficient of neighboring cells under different conditions were normalized by value of the control samples, i.e., ICM treated by only 1/20 min−1 TNF-α. The error bars represent standard deviation of the correlation coefficients of NF-κB oscillation under different experimental conditions.