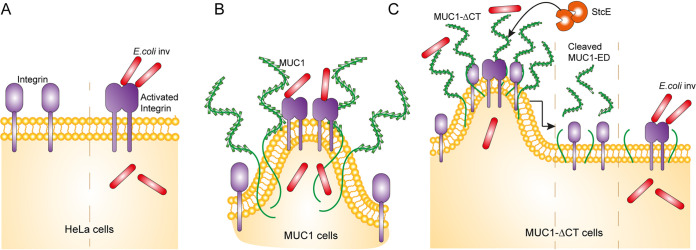
FIG 6.
Working model describing the impact of MUC1 on ITGB1-mediated E. coli inv invasion. (A) In HeLa cells, interaction of E. coli inv with ITGB1 initiates uptake of bacteria. (B) Working model of E. coli inv interaction with ITGB1 in HeLa cells expressing MUC1. MUC1 does not form a barrier that prevents bacterial invasion. The MUC1 extracellular domain alters the cell membrane to tubulated morphology and reorganizes ITGB1, which may reduce overall integrin-bacteria binding, but primes integrin clustering. In this way, expression of MUC1 promotes bacterial uptake instead of being a barrier. (C) Working model of E. coli inv interaction with ITGB1 in HeLa cells expressing MUC1 without the cytoplasmic tail (MUC1-ΔCT). The presence of the MUC1 ED alters the cell membrane to tubulated morphology and reorganizes ITGB1, which may reduce overall integrin-bacteria binding. In the absence of a functional CT, priming and stabilization of integrin clusters will not occur, and this leads to severely reduced bacterial uptake. StcE treatment of MUC1-ΔCT cells removes the ED and reduces the tubulated morphology of the membrane, which leads to recovery of overall integrin-bacteria binding, ITGB1 outside-in signaling, and restored bacterial uptake.