Fig. 3.
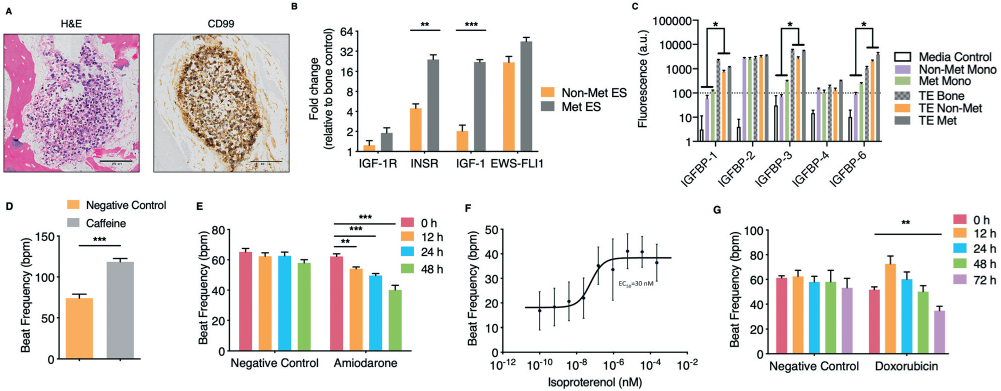
Development and validation of the engineered human Ewing sarcoma (ES) bone tumor and human cardiac tissue. A. Immunohistochemistry analysis of the engineered tumor tissues. H&E staining demonstrates tumor morphology within the tissue engineered bone, and positivity for ES marker CD99. Scale bars: 100 μm. B. Gene expression of ES translocation marker EWS-FLI1 and linsitinib targets in non-metastatic and metastatic ES engineered tissues. Levels were normalized first to β actin and subsequently to the tissue engineered bone control (mean ± s.d., n = 3). C. Proteomic analysis of IGF-1 binding proteins secreted by tumor cells grown in monolayer as compared to our engineered bone (control) and bone tumor tissues (mean ± s.d., n = 3). D. Human engineered cardiac tissue response to caffeine (50 mM) (mean ± s.e.m., n = 5). E. Human engineered cardiac tissue response to amiodarone (2.418 μM) over 48 hours (mean ± s.e.m., n = 6 for negative control; n = 7 for amiodarone). F. Isoproterenol dose-response study of engineered cardiac tissues (mean ± s.e.m., n = 63). G. Response of cardiac tissues to doxorubicin (1 μM) over 72 hours (mean ± s.e.m., n = 7). *P < 0.05; **P < 0.01; ***P < 0.001, by two-way ANOVA with Bonferroni post-test or unpaired, two-tailed Student's t test.
