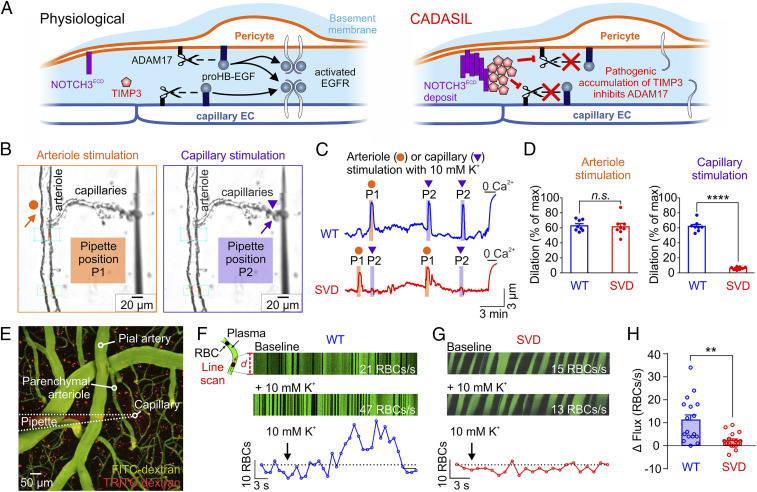
Fig. 1.
Capillary-to-arteriole electrical signaling during functional hyperemia is abrogated in SVD. (A) Proposed mechanism for pathogenic blunting of EGFR activation by accumulation of TIMP3 in CADASIL at the capillary level. (B) Pipette positions for arteriole stimulation (Left, orange arrow) and capillary stimulation (Right, purple arrow) in CaPA preparations. (C) Representative traces of arteriolar diameter showing effects of pressure ejection of 10 mM K+ onto capillaries (P2, purple triangle) on the diameter of upstream arteriolar segments in control preparations from WT and SVD mice. (D) Summary data from 8 WT and 8 SVD mice (n.s., not significant; ****P < 0.0001, unpaired Student’s t test). (E) Micrograph displaying a micropipette containing 10 mM K+ and TRITC-dextran (red) in close apposition to a capillary (FITC-dextran, green) in SVD mice. RBC flux induced by local ejection of K+ onto the capillary was measured by high-frequency line scanning. (F and G) (Top Panels) Raw recordings showing RBC flux at baseline and after application of 10 mM K+ onto a capillary in WT (F) and SVD (G) mice. RBCs appear as black streaks in plasma (green). x axis, time; y axis, scanned capillary distance, d. (Bottom) Full trace from raw recordings shown above. (H) Summary data showing that K+-evoked hyperemia is crippled in SVD mice (n = 16–17 experiments in seven to eight mice; **P < 0.01, unpaired Student’s t test).