Figure 1 |. The cartoon depicts a simplified scheme of the assembly and extension of HS-GAG chains on a PG core protein.
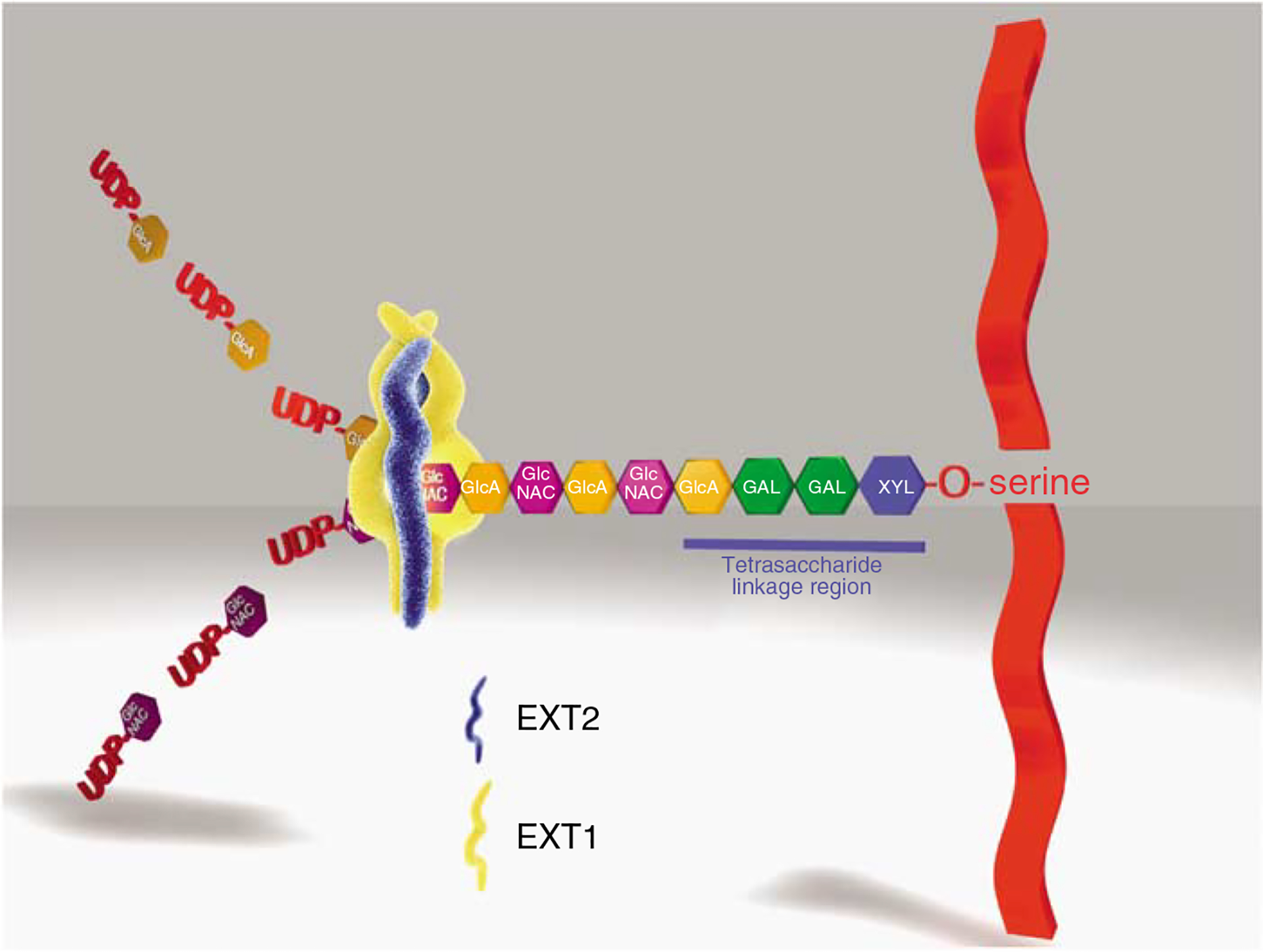
The initial step in the assembly of the HS-GAG chain (and common to all HS and chondroitin sulfate-bearing PGs) is the assembly of a linker tetrasaccharide (glucuronosyl-β 1,3-galactosyl-β 1,3-galactosyl-β 1,4 xylose) attached to a serine residue present in the proteoglycan core protein. Each carbohydrate residue in the linkage region is assembled by a specific glycosyltransferase. In the case of HS-GAG, following the assembly of the linkage region, an N-acetyl glucosamine residue (α-GlcNAC) is the next carbohydrate residue attached to the linkage region by the enzyme EXTL3. Once assembled, the pentasaccharide is recognized by heparan sulfate copolymerase, EXT1/EXT2. The HS copolymerase extends the nascent GAG chain by the sequential addition of a glucuronic acid (UA) and N-acetyl glucosamine (GLN), which are derived from UDP precursors, forming a repetitive copolymer of these subunits (GlcNAcα1–4GlcAβ1–4)n. For the sake of simplification, the subsequent events in the process, such as deacetylation, sulfation, and uronic acid epimerization, which occur during HS chain elongation are not depicted.
