Extended Data Figure 5. Primary and secondary epitopes of class 2 hNAbs.
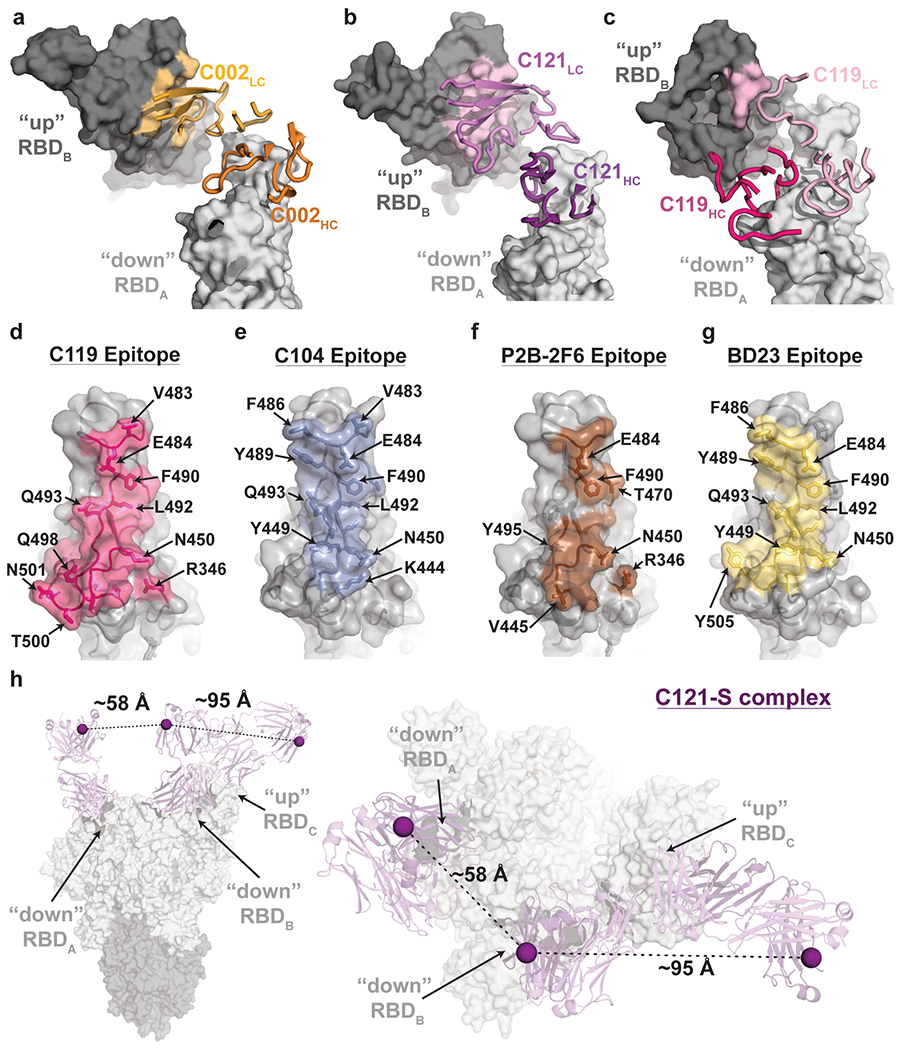
a-c, Primary epitopes for C002 (panel a), C121 (panel b), and C119 (panel c) on “down” RBD. A secondary epitope is observed if a Fab is bound to an adjacent “up” RBD for these NAbs. Antibody paratopes are represented as cartoons. A similar interaction in the C104-S structure is not shown due to low local resolution on the “up” RBD. d-g, Primary epitopes for C119 (panel d), C104 (panel e), P2B-2F6 (panel f; PDB 7BWJ), and BD23 (panel g, PDB 7BYR). The existence of secondary epitopes for P2B-2F6 and BD23 cannot be determined because the P2B-2F6 epitope was determined from a crystal structure with an RBD27, and the BD23-S cryo-EM structure showed only one bound Fab13. h, Measurement of C distance between the C-termini of adjacent C121 CH1 domains (residue 222HC on each Fab). Measurements of this type were used to evaluate whether intra-spike crosslinking by an IgG binding to a single spike trimer was possible for hNAbs in Extended Data Table 1.
